Considerations for modelling diffuse high-grade gliomas and developing clinically relevant therapies
- PMID: 37004686
- PMCID: PMC10348989
- DOI: 10.1007/s10555-023-10100-7
Considerations for modelling diffuse high-grade gliomas and developing clinically relevant therapies
Abstract
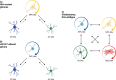
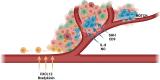
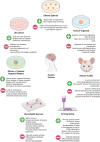
Diffuse high-grade gliomas contain some of the most dangerous human cancers that lack curative treatment options. The recent molecular stratification of gliomas by the World Health Organisation in 2021 is expected to improve outcomes for patients in neuro-oncology through the development of treatments targeted to specific tumour types. Despite this promise, research is hindered by the lack of preclinical modelling platforms capable of recapitulating the heterogeneity and cellular phenotypes of tumours residing in their native human brain microenvironment. The microenvironment provides cues to subsets of glioma cells that influence proliferation, survival, and gene expression, thus altering susceptibility to therapeutic intervention. As such, conventional in vitro cellular models poorly reflect the varied responses to chemotherapy and radiotherapy seen in these diverse cellular states that differ in transcriptional profile and differentiation status. In an effort to improve the relevance of traditional modelling platforms, recent attention has focused on human pluripotent stem cell-based and tissue engineering techniques, such as three-dimensional (3D) bioprinting and microfluidic devices. The proper application of these exciting new technologies with consideration of tumour heterogeneity and microenvironmental interactions holds potential to develop more applicable models and clinically relevant therapies. In doing so, we will have a better chance of translating preclinical research findings to patient populations, thereby addressing the current derisory oncology clinical trial success rate.
Keywords: 3D printing; Clinically relevant; Glioma stem cells; High-grade glioma; Microfluidics; Organoids; Preclinical models; Tissue engineering; Tumour microenvironment.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B, et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA. 2017;318(23):2306–2316. doi: 10.1001/jama.2017.18718. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

