Estimating the Prevalence of over- and Under-Reporting in HIV Testing, Status and Treatment in Rural Northeast South Africa: A Comparison of a Survey and Clinic Records
- PMID: 37004687
- PMCID: PMC10066974
- DOI: 10.1007/s10461-023-04045-9
Estimating the Prevalence of over- and Under-Reporting in HIV Testing, Status and Treatment in Rural Northeast South Africa: A Comparison of a Survey and Clinic Records
Abstract
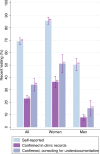
We assess the accuracy of self-reported testing, HIV status, and treatment responses compared to clinical records in Ehlanzeni District, South Africa. We linked a 2018 population-based survey of adults 18-49 years old with clinical data at local primary healthcare facilities from 2014 to 2018. We calculated self-reported testing, HIV status, and treatment, and triangulated findings with clinic record data. We adjusted testing estimates for known gaps in HIV test documentation. Of 2089 survey participants, 1657 used a study facility and were eligible for analysis. Half of men and 84% of women reported an HIV test in the past year. One third of reported tests could be confirmed in clinic data within 1 year and an additional 13% within 2 years; these fractions increased to 57% and 22% respectively limiting to participants with a verified clinic file. After accounting for gaps in clinic documentation, we found that prevalence of recent HIV testing was closer to 15% among men and 51% in women. Estimated prevalence of known HIV was 16.2% based on self-report vs. 27.6% with clinic documentation. Relative to clinical records among confirmed clinic users, self report of HIV testing and of current treatment were highly sensitive but non-specific (sensitivity 95.5% and 98.8%, specificity 24.2% and 16.1% respectively), while self report of HIV status was highly specific but not sensitive (sensitivity 53.0%, specificity 99.3%). While clinical records are imperfect, survey-based measures should be interpreted with caution in this rural South African setting.
Keywords: HIV testing; South Africa; self-reported measures; survey research.
© 2023. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Dr. Leslie reports research funding from the National Institutes of Health, the Bill & Melinda Gates Foundation, and the InterAmerican Development Bank during the course of this research. The authors report no conflicts of interest.
Figures
References
-
- Let Our Actions Count. : South Africa’s National Strategic Plan for HIV, TB and STIs 2017–2022 [Internet]. Pretoria, South Africa: National Department of Health; 2017 May [cited 2022 Apr 4]. Available from: https://www.gov.za/sites/default/files/gcis_document/201705/nsp-hiv-tb-s...
-
- National Department of Health. National Consolidated Guidelines for the Management of HIV in Adults, Adolescents, Children and Infants and Prevention of Mother-to-Child Transmission [Internet]. South Africa: National Department of Health. ; 2020 Feb [cited 2023 Jan 31]. Available from: https://sahivsoc.org/Files/National%20Consolidated%20Guidelines%20300620...
-
- Baggaley RF, Hollingsworth TD. How universal does universal test and treat have to be? The Lancet HIV. 2020 May 1;7(5):e306–8. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous