Recombinant polio-rhinovirus immunotherapy for recurrent paediatric high-grade glioma: a phase 1b trial
- PMID: 37004712
- PMCID: PMC11104482
- DOI: 10.1016/S2352-4642(23)00031-7
Recombinant polio-rhinovirus immunotherapy for recurrent paediatric high-grade glioma: a phase 1b trial
Abstract
Background: Outcomes of recurrent paediatric high-grade glioma are poor, with a median overall survival of less than 6 months. Viral immunotherapy, such as the polio-rhinovirus chimera lerapolturev, is a novel approach for treatment of recurrent paediatric high-grade glioma and has shown promise in adults with recurrent glioblastoma. The poliovirus receptor CD155 is ubiquitously expressed in malignant paediatric brain tumours and is a treatment target in paediatric high-grade glioma. We aimed to assess the safety of lerapolturev when administered as a single dose intracerebrally by convection enhanced delivery in children and young people with recurrent WHO grade 3 or grade 4 glioma, and to assess overall survival in these patients.
Methods: This phase 1b trial was done at the Duke University Medical Center (Durham, NC, USA). Patients aged 4-21 years with recurrent high-grade malignant glioma (anaplastic astrocytoma, glioblastoma, anaplastic oligoastrocytoma, anaplastic oligodendroglioma, or anaplastic pleomorphic xanthoastrocytoma) or anaplastic ependymoma, atypical teratoid rhabdoid tumour, or medulloblastoma with infusible disease were eligible for this study. A catheter was tunnelled beneath the scalp for a distance of at least 5 cm to aid in prevention of infection. The next day, lerapolturev at a dose of 5 × 107 median tissue culture infectious dose in 3 mL infusate loaded in a syringe was administered via a pump at a rate of 0·5 mL per h as a one-time dose. The infusion time was approximately 6·5 h to compensate for volume of the tubing. The primary endpoint was the proportion of patients with unacceptable toxic effects during the 14-day period after lerapolturev treatment. The study is registered with ClinicalTrials.gov, NCT03043391.
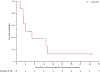
Findings: Between Dec 5, 2017, and May 12, 2021, 12 patients (11 unique patients) were enrolled in the trial. Eight patients were treated with lerapolturev. The median patient age was 16·5 years (IQR 11·0-18·0), five (63%) of eight patients were male and three (38%) were female, and six (75%) of eight patients were White and two (25%) were Black or African American. The median number of previous chemotherapeutic regimens was 3·50 (IQR 1·25-5·00). Six of eight patients had 26 treatment-related adverse events attributable to lerapolturev. There were no irreversible (ie, persisted longer than 2 weeks) treatment-related grade 4 adverse events or deaths. Treatment-related grade 3 adverse events included headaches in two patients and seizure in one patient. Four patients received low-dose bevacizumab on-study for treatment-related peritumoural inflammation or oedema, diagnosed by both clinical symptoms plus fluid-attenuated inversion recovery MRI. The median overall survival was 4·1 months (95% CI 1·2-10·1). One patient remains alive after 22 months.
Interpretation: Convection enhanced delivery of lerapolturev is safe enough in the treatment of recurrent paediatric high-grade glioma to proceed to the next phase of trial.
Funding: Solving Kids Cancer, B+ Foundation, Musella Foundation, and National Institutes of Health.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests MG, HSF, DPB, AD, DDB, and DMA are equity shareholders of Istari Oncology, the company licensing lerapolturev. MCB, MG, DDB, and DMA own intellectual property licensed to Istari Oncology. MCB, MG, DDB, HSF, and AD received consultancy fees from Istari Oncology. EMT is a scientific consultant for Oncoheroes Biosciences. All other authors declare no competing interests.
Figures
Similar articles
-
Recurrent Glioblastoma Treated with Recombinant Poliovirus.N Engl J Med. 2018 Jul 12;379(2):150-161. doi: 10.1056/NEJMoa1716435. Epub 2018 Jun 26. N Engl J Med. 2018. PMID: 29943666 Free PMC article. Clinical Trial.
-
Chronic convection-enhanced delivery of topotecan for patients with recurrent glioblastoma: a first-in-patient, single-centre, single-arm, phase 1b trial.Lancet Oncol. 2022 Nov;23(11):1409-1418. doi: 10.1016/S1470-2045(22)00599-X. Epub 2022 Oct 13. Lancet Oncol. 2022. PMID: 36243020 Free PMC article. Clinical Trial.
-
Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial.Lancet Oncol. 2019 Jul;20(7):1011-1022. doi: 10.1016/S1470-2045(19)30277-3. Epub 2019 May 28. Lancet Oncol. 2019. PMID: 31151904 Free PMC article. Clinical Trial.
-
Recurrent malignant glioma in adults.Curr Treat Options Oncol. 2002 Dec;3(6):509-24. doi: 10.1007/s11864-002-0070-8. Curr Treat Options Oncol. 2002. PMID: 12392640 Review.
-
Molecular Genetics and Targeted Therapies for Paediatric High-grade Glioma.Cancer Genomics Proteomics. 2022 Jul-Aug;19(4):390-414. doi: 10.21873/cgp.20328. Cancer Genomics Proteomics. 2022. PMID: 35732328 Free PMC article. Review.
Cited by
-
Advances in oncolytic herpes simplex virus and adenovirus therapy for recurrent glioma.Front Immunol. 2023 Nov 2;14:1285113. doi: 10.3389/fimmu.2023.1285113. eCollection 2023. Front Immunol. 2023. PMID: 38022620 Free PMC article. Review.
-
A phase I study of convection-enhanced delivery (CED) of liposomal-irinotecan using real-time magnetic resonance imaging in patients with recurrent high-grade glioma.J Neurooncol. 2025 Mar;172(1):219-227. doi: 10.1007/s11060-024-04904-y. Epub 2025 Jan 6. J Neurooncol. 2025. PMID: 39760796 Free PMC article. Clinical Trial.
-
Single-cell sequencing uncovers the mechanistic role of DAPK1 in glioma and its diagnostic and prognostic implications.Front Immunol. 2025 Jan 24;15:1463747. doi: 10.3389/fimmu.2024.1463747. eCollection 2024. Front Immunol. 2025. PMID: 39926603 Free PMC article.
-
Focus on current and emerging treatment options for glioma: A comprehensive review.World J Clin Oncol. 2024 Apr 24;15(4):482-495. doi: 10.5306/wjco.v15.i4.482. World J Clin Oncol. 2024. PMID: 38689623 Free PMC article. Review.
-
Convection enhanced delivery of Rhenium (186Re) Obisbemeda (186RNL) in recurrent glioma: a multicenter, single arm, phase 1 clinical trial.Nat Commun. 2025 Mar 7;16(1):2079. doi: 10.1038/s41467-025-57263-1. Nat Commun. 2025. PMID: 40055350 Free PMC article. Clinical Trial.
References
-
- US Centers for Disease Control and Prevention. National Center for Health Statistics. Declines in cancer death rates among children and adolescents in the United States, 1999–2014. September, 2016. http://www.cdc.gov/nchs/products/databriefs/db257.htm (accessed Oct 11, 2016).
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials