Proarrhythmic toxicity of low dose bisphenol A and its analogs in human iPSC-derived cardiomyocytes and human cardiac organoids through delay of cardiac repolarization
- PMID: 37004823
- PMCID: PMC10121900
- DOI: 10.1016/j.chemosphere.2023.138562
Proarrhythmic toxicity of low dose bisphenol A and its analogs in human iPSC-derived cardiomyocytes and human cardiac organoids through delay of cardiac repolarization
Abstract
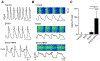
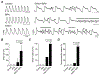
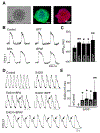
Bisphenol A (BPA) and its analogs are common environmental chemicals with many potential adverse health effects. The impact of environmentally relevant low dose BPA on human heart, including cardiac electrical properties, is not understood. Perturbation of cardiac electrical properties is a key arrhythmogenic mechanism. In particular, delay of cardiac repolarization can cause ectopic excitation of cardiomyocytes and malignant arrhythmia. This can occur as a result of genetic mutations (i.e., long QT (LQT) syndrome), or cardiotoxicity of drugs and environmental chemicals. To define the impact of low dose BPA on electrical properties of cardiomyocytes in a human-relevant model system, we examined the rapid effects of 1 nM BPA in human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) using patch-clamp and confocal fluorescence imaging. Acute exposure to BPA delayed repolarization and prolonged action potential duration (APD) in hiPSC-CMs through inhibition of the hERG K+ channel. In nodal-like hiPSC-CMs, BPA acutely increased pacing rate through stimulation of the If pacemaker channel. Existing arrhythmia susceptibility determines the response of hiPSC-CMs to BPA. BPA resulted in modest APD prolongation but no ectopic excitation in baseline condition, while rapidly promoted aberrant excitations and tachycardia-like events in myocytes that had drug-simulated LQT phenotype. In hiPSC-CM-based human cardiac organoids, the effects of BPA on APD and aberrant excitation were shared by its analog chemicals, which are often used in "BPA-free" products, with bisphenol AF having the largest effects. Our results reveal that BPA and its analogs have repolarization delay-associated pro-arrhythmic toxicity in human cardiomyocytes, particularly in myocytes that are prone to arrhythmias. The toxicity of these chemicals depends on existing pathophysiological conditions of the heart, and may be particularly pronounced in susceptible individuals. An individualized approach is needed in risk assessment and protection.
Keywords: Arrhythmia; Bisphenol A; Human cardiac organoid; Human iPSC-CMs; Long QT syndrome.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
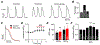
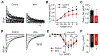
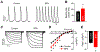
References
-
- Bezzina CR, Lahrouchi N, Priori SG, 2015. Genetics of sudden cardiac death. Circ Res 116, 1919–1936. - PubMed
-
- Cai S, Rao X, Ye J, Ling Y, Mi S, Chen H, Fan C, Li Y, 2020. Relationship between urinary bisphenol a levels and cardiovascular diseases in the U.S. adult population, 2003-2014. Ecotoxicol Environ Saf 192, 110300. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

