Efficacy of vitamin D3 supplementation on cancer mortality: Systematic review and individual patient data meta-analysis of randomised controlled trials
- PMID: 37004841
- PMCID: PMC10214278
- DOI: 10.1016/j.arr.2023.101923
Efficacy of vitamin D3 supplementation on cancer mortality: Systematic review and individual patient data meta-analysis of randomised controlled trials
Abstract
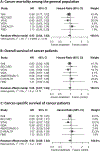
To evaluate the effect of vitamin D3 supplementation on cancer mortality in the general population and on prognosis in cancer patients, a systematic review and meta-analysis of randomised, placebo-controlled trials (RCTs) and individual patient data (IPD) was conducted. Overall, 14 RCTs with a total of 104,727 participants (2015 cancer deaths) were identified and 7 RCTs, including 90 % of all study participants (n = 94,068), could be included in the IPD meta-analyses. The main meta-analysis of the 14 RCTs yielded a statistically non-significant reduction in cancer mortality by 6 % (risk ratio (RR) [95%-confidence interval (95%CI)]: 0.94 [0.86-1.02]). Subgroup analyses revealed a 12 % lower cancer mortality in the vitamin D3 group compared with the placebo group in 10 trials with a daily dosing regimen (RR [95%CI]: 0.88 [0.78-0.98]), whereas no mortality reduction was seen in 4 trials using a bolus regimen (RR [95%CI]: 1.07 [0.91-1.24]; p-value for interaction: 0.042). The IPD meta-analysis (RR [95%CI]: 0.93 [0.84; 1.02]) confirmed the finding of all trials. The IPD were used to test effect modification by age, sex, body mass index, ethnicity, baseline serum 25-hydroxyvitamin D concentration, adherence and cancer-related factors but no statistically significant findings were obtained in meta-analyses of all trials. When restricted to trials with daily dosing in a post-hoc analysis, adults aged ≥ 70 years (RR [95%CI]: 0.83 [0.77; 0.98]) and subjects with vitamin D3 therapy initiation before cancer diagnosis (RR [95%CI]: 0.87 [0.69; 0.99]) appeared to benefit most from daily vitamin D3 supplementation. Measurements of baseline 25-hydroxyvitamin D levels and inclusion of other than non-Hispanic White adults were too sparse in the trials to draw conclusions. Results for all-cause and cancer-specific survival of participants with cancer were comparable to those obtained in the general population for cancer mortality. In conclusion, vitamin D3 did not reduce cancer mortality in the main meta-analysis of all RCTs because the observed risk reduction by 6 % was not statistically significant. However, a subgroup analysis revealed that vitamin D3 administered daily, in contrast to bolus supplementation, reduced cancer mortality by 12 %.
Keywords: Cancer; Individual patient-data; Mortality; Survival; Systematic review; Vitamin D.
Copyright © 2023 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years except for Julie E. Buring who declares an association to Pharmavite. All authors further declare no other relationships or activities that could appear to have influenced the submitted work.
Figures

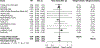



References
-
- Akiba T, Morikawa T, Odaka M, Nakada T, Kamiya N, Yamashita M, Yabe M, Inagaki T, Asano H, Mori S, Tsukamoto Y, Urashima M, 2018. Vitamin D supplementation and survival of patients with non-small cell lung cancer: a randomized, double-blind, placebo-controlled trial. Clin. Cancer Res. 24, 4089–4097. - PubMed
-
- Avenell A, MacLennan GS, Jenkinson DJ, McPherson GC, McDonald AM, Pant PR, Grant AM, Campbell MK, Anderson FH, Cooper C, Francis RM, Gillespie WJ, Robinson CM, Torgerson DJ, Wallace WA, 2012. Long-term follow-up for mortality and cancer in a randomized placebo-controlled trial of vitamin D3 and/or calcium (RECORD Trial). J. Clin. Endocrinol. Metab. 97, 614–622. - PubMed
-
- Baron JA, Barry EL, Mott LA, Rees JR, Sandler RS, Snover DC, Bostick RM, Ivanova A, Cole BF, Ahnen DJ, Beck GJ, Bresalier RS, Burke CA, Church TR, Cruz-Correa M, Figueiredo JC, Goodman M, Kim AS, Robertson DJ, Rothstein R, Shaukat A, Seabrook ME, Summers RW, 2015. A trial of calcium and vitamin d for the prevention of colorectal adenomas. N. Engl. J. Med. 373, 1519–1530. - PMC - PubMed

