Targeted dexamethasone nano-prodrug for corneal neovascularization management
- PMID: 37004870
- PMCID: PMC10826162
- DOI: 10.1016/j.bj.2023.03.005
Targeted dexamethasone nano-prodrug for corneal neovascularization management
Abstract
Background: To overcome the drawbacks of traditional therapy for corneal neovascularization (CNV), we evaluated the efficacy of polyethylene glycol (PEG)-conjugated Ala-Pro-Arg-Pro-Gly (APRPG) peptide modified dexamethasone (Dex), a novel nano-prodrug (Dex-PEG-APRPG, DPA).
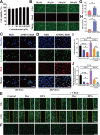
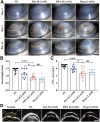
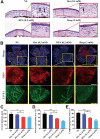
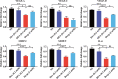
Methods: Characterization of DPA nano-prodrug were measured with transmission electron microscopy (TEM) and dynamic light scattering (DLS) analyses. Cytotoxicity and effects on cell migration and tube formation of DPA were evaluated in vitro. A murine CNV model was established by cornea alkali burn. The injured corneas were given eye drops of DPA (0.2 mM), Dex solution (0.2 mM), Dexp (2 mM), or normal saline three times a day. After two weeks, eyes were obtained for the analysis of histopathology, immunostaining, and mRNA expression.
Results: DPA with an average diameter of 30 nm, presented little cytotoxicity and had good ocular biocompatibility. More importantly, DPA showed specific targeting to vascular endothelial cells with efficient inhibition on cell migration and tube formation. In a mouse CNV model, clinical, histological, and immunohistochemical examination results revealed DPA had a much stronger angiogenesis suppression than Dex, resembling a clinical drug with an order of magnitude higher concentration. This was ascribed to the significant downregulations in the expression of pro-angiogenic and pro-inflammatory factors in the corneas. In vivo imaging results also demonstrated that APRPG could prolong ocular retention time.
Conclusions: This study suggests that DPA nano-prodrug occupies advantages of specific targeting ability and improved bioavailability over conventional therapy, and holds great potential for safe and efficient CNV therapy.
Keywords: Angiogenic vessel-homing peptide; Corneal neovascularization; Dexamethasone; Nano-prodrug; Targeted drug delivery.
© 2023 Chang Gung University. Publishing services provided by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Conflict of interest statement
Conflicts of interest The authors declare that they have no conflicts of interest.
Figures



Similar articles
-
Treatment Options for Alkali Burn-Induced Corneal Neovascularization: A Comparative Analysis of Two Tyrosine Kinase Inhibitors.Cornea. 2025 May 27;44(9):1174-1181. doi: 10.1097/ICO.0000000000003890. Cornea. 2025. PMID: 40424526
-
Topical pharmacologic interventions versus placebo for epidemic keratoconjunctivitis.Cochrane Database Syst Rev. 2022 Mar 3;3(3):CD013520. doi: 10.1002/14651858.CD013520.pub2. Cochrane Database Syst Rev. 2022. PMID: 35238405 Free PMC article.
-
Adjunctive steroid therapy versus antibiotics alone for acute endophthalmitis after intraocular procedure.Cochrane Database Syst Rev. 2017 Feb 22;2(2):CD012131. doi: 10.1002/14651858.CD012131.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2022 Jun 6;6:CD012131. doi: 10.1002/14651858.CD012131.pub3. PMID: 28225198 Free PMC article. Updated.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Amniotic membrane transplantation for acute ocular burns.Cochrane Database Syst Rev. 2022 Sep 1;9(9):CD009379. doi: 10.1002/14651858.CD009379.pub3. Cochrane Database Syst Rev. 2022. PMID: 36047788 Free PMC article.
Cited by
-
Subconjunctival conbercept for the treatment of corneal neovascularization.Int J Ophthalmol. 2023 Jun 18;16(6):871-875. doi: 10.18240/ijo.2023.06.06. eCollection 2023. Int J Ophthalmol. 2023. PMID: 37332556 Free PMC article.
-
Glucocorticoid Receptor Signaling Is Critical for Mouse Corneal Development, Inhibition of Inflammatory Response, and Neovascularization of the Cornea.Am J Pathol. 2024 Oct;194(10):1938-1950. doi: 10.1016/j.ajpath.2024.06.005. Am J Pathol. 2024. PMID: 39322334
-
Progress in Nanotechnology for Treating Ocular Surface Chemical Injuries: Reflecting on Advances in Ophthalmology.Adv Sci (Weinh). 2025 Feb;12(6):e2407340. doi: 10.1002/advs.202407340. Epub 2025 Jan 4. Adv Sci (Weinh). 2025. PMID: 39755928 Free PMC article. Review.
References
-
- Chan PS, Li Q, Zhang B, To KKW, Leung SSY. In vivo biocompatibility and efficacy of dexamethasone-loaded PLGA-PEG-PLGA thermogel in an alkali-burn induced corneal neovascularization disease model. Eur J Pharm Biopharm. 2020;155:190–198. - PubMed
-
- Han H, Li S, Xu M, Zhong Y, Fan W, Xu J, et al. Polymer- and lipid-based nanocarriers for ocular drug delivery: Current status and future perspectives. Adv Drug Deliv Rev. 2023;196:114770. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

