Serum amyloid A augments the atherogenic effects of cholesteryl ester transfer protein
- PMID: 37004910
- PMCID: PMC10165456
- DOI: 10.1016/j.jlr.2023.100365
Serum amyloid A augments the atherogenic effects of cholesteryl ester transfer protein
Abstract
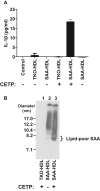
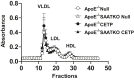
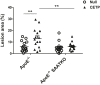
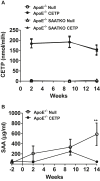
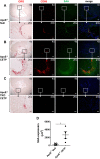
Serum amyloid A (SAA) is predictive of CVD in humans and causes atherosclerosis in mice. SAA has many proatherogenic effects in vitro. However, HDL, the major carrier of SAA in the circulation, masks these effects. The remodeling of HDL by cholesteryl ester transfer protein (CETP) liberates SAA restoring its proinflammatory activity. Here, we investigated whether deficiency of SAA suppresses the previously described proatherogenic effect of CETP. ApoE-/- mice and apoE-/- mice deficient in the three acute-phase isoforms of SAA (SAA1.1, SAA2.1, and SAA3; "apoE-/- SAA-TKO") with and without adeno-associated virus-mediated expression of CETP were studied. There was no effect of CETP expression or SAA genotype on plasma lipids or inflammatory markers. Atherosclerotic lesion area in the aortic arch of apoE-/- mice was 5.9 ± 1.2%; CETP expression significantly increased atherosclerosis in apoE-/- mice (13.1 ± 2.2%). However, atherosclerotic lesion area in the aortic arch of apoE-/- SAA-TKO mice (5.1 ± 1.1%) was not significantly increased by CETP expression (6.2 ± 0.9%). The increased atherosclerosis in apoE-/- mice expressing CETP was associated with markedly increased SAA immunostaining in aortic root sections. Thus, SAA augments the atherogenic effects of CETP, which suggests that inhibiting CETP may be of particular benefit in patients with high SAA.
Keywords: HDL; apolipoprotein; atherosclerosis; cholesteryl ester transfer protein; inflammation; lipid metabolism; serum amyloid A.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures
References
-
- Uhlar C.M., Burgess C.J., Sharp P.M., Whitehead A.S. Evolution of the serum amyloid A (SAA) protein superfamily. Genomics. 1994;19:228–235. - PubMed
-
- Uhlar C.M., Whitehead A.S. Serum amyloid A, the major vertebrate acute-phase reactant. Eur. J. Biochem. 1999;265:501–523. - PubMed
-
- Eklund K.K., Niemi K., Kovanen P.T. Immune functions of serum amyloid A. Crit. Rev. Immunol. 2012;32:335–348. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

