Aryl hydrocarbon receptor maintains hepatic mitochondrial homeostasis in mice
- PMID: 37004989
- PMCID: PMC10106517
- DOI: 10.1016/j.molmet.2023.101717
Aryl hydrocarbon receptor maintains hepatic mitochondrial homeostasis in mice
Abstract
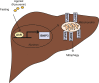
Objective: Mitophagy removes damaged mitochondria to maintain cellular homeostasis. Aryl hydrocarbon receptor (AhR) expression in the liver plays a crucial role in supporting normal liver functions, but its impact on mitochondrial function is unclear. Here, we identified a new role of AhR in the regulation of mitophagy to control hepatic energy homeostasis.
Methods: In this study, we utilized primary hepatocytes from AhR knockout (KO) mice and AhR knockdown AML12 hepatocytes. An endogenous AhR ligand, kynurenine (Kyn), was used to activate AhR in AML12 hepatocytes. Mitochondrial function and mitophagy process were comprehensively assessed by MitoSOX and mt-Keima fluorescence imaging, Seahorse XF-based oxygen consumption rate measurement, and Mitoplate S-1 mitochondrial substrate utilization analysis.
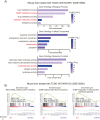
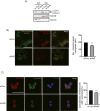
Results: Transcriptomic analysis indicated that mitochondria-related gene sets were dysregulated in AhR KO liver. In both primary mouse hepatocytes and AML12 hepatocyte cell lines, AhR inhibition strongly suppressed mitochondrial respiration rate and substrate utilization. AhR inhibition also blunted the fasting response of several essential autophagy genes and the mitophagy process. We further identified BCL2 interacting protein 3 (BNIP3), a mitophagy receptor that senses nutrient stress, as an AhR target gene. AhR is directly recruited to the Bnip3 genomic locus, and Bnip3 transcription was enhanced by AhR endogenous ligand treatment in wild-type liver and abolished entirely in AhR KO liver. Mechanistically, overexpression of Bnip3 in AhR knockdown cells mitigated the production of mitochondrial reactive oxygen species (ROS) and restored functional mitophagy.
Conclusions: AhR regulation of the mitophagy receptor BNIP3 coordinates hepatic mitochondrial function. Loss of AhR induces mitochondrial ROS production and impairs mitochondrial respiration. These findings provide new insight into how endogenous AhR governs hepatic mitochondrial homeostasis.
Keywords: Autophagy; BNIP3; Kynurenine; Mitophagy; Reactive oxygen species.
Copyright © 2023 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

