Patients with ACPA-positive and ACPA-negative rheumatoid arthritis show different serological autoantibody repertoires and autoantibody associations with disease activity
- PMID: 37005480
- PMCID: PMC10066987
- DOI: 10.1038/s41598-023-32428-4
Patients with ACPA-positive and ACPA-negative rheumatoid arthritis show different serological autoantibody repertoires and autoantibody associations with disease activity
Abstract
Patients with rheumatoid arthritis (RA) can test either positive or negative for circulating anti-citrullinated protein antibodies (ACPA) and are thereby categorized as ACPA-positive (ACPA+) or ACPA-negative (ACPA-), respectively. In this study, we aimed to elucidate a broader range of serological autoantibodies that could further explain immunological differences between patients with ACPA+ RA and ACPA- RA. On serum collected from adult patients with ACPA+ RA (n = 32), ACPA- RA (n = 30), and matched healthy controls (n = 30), we used a highly multiplex autoantibody profiling assay to screen for over 1600 IgG autoantibodies that target full-length, correctly folded, native human proteins. We identified differences in serum autoantibodies between patients with ACPA+ RA and ACPA- RA compared with healthy controls. Specifically, we found 22 and 19 autoantibodies with significantly higher abundances in ACPA+ RA patients and ACPA- RA patients, respectively. Among these two sets of autoantibodies, only one autoantibody (anti-GTF2A2) was common in both comparisons; this provides further evidence of immunological differences between these two RA subgroups despite sharing similar symptoms. On the other hand, we identified 30 and 25 autoantibodies with lower abundances in ACPA+ RA and ACPA- RA, respectively, of which 8 autoantibodies were common in both comparisons; we report for the first time that the depletion of certain autoantibodies may be linked to this autoimmune disease. Functional enrichment analysis of the protein antigens targeted by these autoantibodies showed an over-representation of a range of essential biological processes, including programmed cell death, metabolism, and signal transduction. Lastly, we found that autoantibodies correlate with Clinical Disease Activity Index, but associate differently depending on patients' ACPA status. In all, we present candidate autoantibody biomarker signatures associated with ACPA status and disease activity in RA, providing a promising avenue for patient stratification and diagnostics.
© 2023. The Author(s).
Conflict of interest statement
Dr. Davis has a research grant from Pfizer and has rights to royalties for technology licensed to Girihlet. Neither Pfizer nor Girihlet had any role in the design or conduct of this study. All other authors do not possess any conflict of interest.
Figures
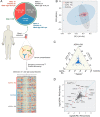

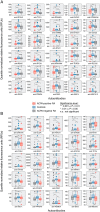

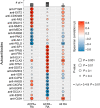
References
-
- Rönnelid J, et al. Longitudinal analysis of citrullinated protein/peptide antibodies (anti-CP) during 5 year follow up in early rheumatoid arthritis: Anti-CP status predicts worse disease activity and greater radiological progression. Ann. Rheum. Dis. 2005;64:1744–1749. doi: 10.1136/ard.2004.033571. - DOI - PMC - PubMed
-
- Burgers LE, van Steenbergen HW, Ten Brinck RM, Huizinga TW, van der Helm-van Mil AH. Differences in the symptomatic phase preceding ACPA-positive and ACPA-negative RA: A longitudinal study in arthralgia during progression to clinical arthritis. Ann. Rheum. Dis. 2017;76:1751–1754. doi: 10.1136/annrheumdis-2017-211325. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

