PIEZO1 and PECAM1 interact at cell-cell junctions and partner in endothelial force sensing
- PMID: 37005489
- PMCID: PMC10067937
- DOI: 10.1038/s42003-023-04706-4
PIEZO1 and PECAM1 interact at cell-cell junctions and partner in endothelial force sensing
Abstract
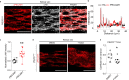
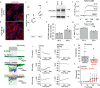
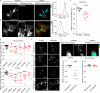
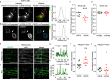
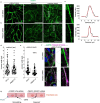
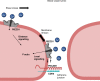
Two prominent concepts for the sensing of shear stress by endothelium are the PIEZO1 channel as a mediator of mechanically activated calcium ion entry and the PECAM1 cell adhesion molecule as the apex of a triad with CDH5 and VGFR2. Here, we investigated if there is a relationship. By inserting a non-disruptive tag in native PIEZO1 of mice, we reveal in situ overlap of PIEZO1 with PECAM1. Through reconstitution and high resolution microscopy studies we show that PECAM1 interacts with PIEZO1 and directs it to cell-cell junctions. PECAM1 extracellular N-terminus is critical in this, but a C-terminal intracellular domain linked to shear stress also contributes. CDH5 similarly drives PIEZO1 to junctions but unlike PECAM1 its interaction with PIEZO1 is dynamic, increasing with shear stress. PIEZO1 does not interact with VGFR2. PIEZO1 is required in Ca2+-dependent formation of adherens junctions and associated cytoskeleton, consistent with it conferring force-dependent Ca2+ entry for junctional remodelling. The data suggest a pool of PIEZO1 at cell junctions, the coming together of PIEZO1 and PECAM1 mechanisms and intimate cooperation of PIEZO1 and adhesion molecules in tailoring junctional structure to mechanical requirement.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

