Tumor microenvironment signaling and therapeutics in cancer progression
- PMID: 37005490
- PMCID: PMC10174093
- DOI: 10.1002/cac2.12416
Tumor microenvironment signaling and therapeutics in cancer progression
Abstract
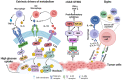
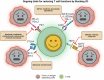
Tumor development and metastasis are facilitated by the complex interactions between cancer cells and their microenvironment, which comprises stromal cells and extracellular matrix (ECM) components, among other factors. Stromal cells can adopt new phenotypes to promote tumor cell invasion. A deep understanding of the signaling pathways involved in cell-to-cell and cell-to-ECM interactions is needed to design effective intervention strategies that might interrupt these interactions. In this review, we describe the tumor microenvironment (TME) components and associated therapeutics. We discuss the clinical advances in the prevalent and newly discovered signaling pathways in the TME, the immune checkpoints and immunosuppressive chemokines, and currently used inhibitors targeting these pathways. These include both intrinsic and non-autonomous tumor cell signaling pathways in the TME: protein kinase C (PKC) signaling, Notch, and transforming growth factor (TGF-β) signaling, Endoplasmic Reticulum (ER) stress response, lactate signaling, Metabolic reprogramming, cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) and Siglec signaling pathways. We also discuss the recent advances in Programmed Cell Death Protein 1 (PD-1), Cytotoxic T-Lymphocyte Associated Protein 4 (CTLA4), T-cell immunoglobulin mucin-3 (TIM-3) and Lymphocyte Activating Gene 3 (LAG3) immune checkpoint inhibitors along with the C-C chemokine receptor 4 (CCR4)- C-C class chemokines 22 (CCL22)/ and 17 (CCL17), C-C chemokine receptor type 2 (CCR2)- chemokine (C-C motif) ligand 2 (CCL2), C-C chemokine receptor type 5 (CCR5)- chemokine (C-C motif) ligand 3 (CCL3) chemokine signaling axis in the TME. In addition, this review provides a holistic understanding of the TME as we discuss the three-dimensional and microfluidic models of the TME, which are believed to recapitulate the original characteristics of the patient tumor and hence may be used as a platform to study new mechanisms and screen for various anti-cancer therapies. We further discuss the systemic influences of gut microbiota in TME reprogramming and treatment response. Overall, this review provides a comprehensive analysis of the diverse and most critical signaling pathways in the TME, highlighting the associated newest and critical preclinical and clinical studies along with their underlying biology. We highlight the importance of the most recent technologies of microfluidics and lab-on-chip models for TME research and also present an overview of extrinsic factors, such as the inhabitant human microbiome, which have the potential to modulate TME biology and drug responses.
Keywords: 3D-model; cancer therapy; gut microbiota; immune signaling; metabolism; signaling; tumor microenvironment.
© 2023 The Authors. Cancer Communications published by John Wiley & Sons Australia, Ltd. on behalf of Sun Yat-sen University Cancer Center.
Conflict of interest statement
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Maman S, Witz IP. A history of exploring cancer in context. Nat Rev Cancer. 2018;18(6):359–76. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. - PubMed
-
- Bejarano L, Jordāo MJC, Joyce JA. Therapeutic Targeting of the Tumor Microenvironment. Cancer Discov. 2021;11(4):933–59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

