SGLT2-inhibitors effects on the coronary fibrous cap thickness and MACEs in diabetic patients with inducible myocardial ischemia and multi vessels non-obstructive coronary artery stenosis
- PMID: 37005586
- PMCID: PMC10067292
- DOI: 10.1186/s12933-023-01814-7
SGLT2-inhibitors effects on the coronary fibrous cap thickness and MACEs in diabetic patients with inducible myocardial ischemia and multi vessels non-obstructive coronary artery stenosis
Abstract
Background: Sodium-glucose transporter 2 inhibitors (SGLT2-I) could modulate atherosclerotic plaque progression, via down-regulation of inflammatory burden, and lead to reduction of major adverse cardiovascular events (MACEs) in type 2 diabetes mellitus (T2DM) patients with ischemic heart disease (IHD). T2DM patients with multivessel non-obstructive coronary stenosis (Mv-NOCS) have over-inflammation and over-lipids' plaque accumulation. This could reduce fibrous cap thickness (FCT), favoring plaque rupture and MACEs. Despite this, there is not conclusive data about the effects of SGLT2-I on atherosclerotic plaque phenotype and MACEs in Mv-NOCS patients with T2DM. Thus, in the current study, we evaluated SGLT2-I effects on Mv-NOCS patients with T2DM in terms of FCT increase, reduction of systemic and coronary plaque inflammation, and MACEs at 1 year of follow-up.
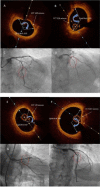
Methods: In a multi-center study, we evaluated 369 T2DM patients with Mv-NOCS divided in 258 (69.9%) patients that did not receive the SGLT2-I therapy (Non-SGLT2-I users), and 111 (30.1%) patients that were treated with SGLT2-I therapy (SGLT2-I users) after percutaneous coronary intervention (PCI) and optical coherence tomography (OCT) evaluation. As the primary study endpoint, we evaluated the effects of SGLT2-I on FCT changes at 1 year of follow-up. As secondary endpoints, we evaluated at baseline and at 12 months follow-up the inflammatory systemic and plaque burden and rate of MACEs, and predictors of MACE through multivariable analysis.
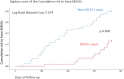
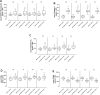
Results: At 6 and 12 months of follow-up, SGLT2-I users vs. Non-SGLT2-I users showed lower body mass index (BMI), glycemia, glycated hemoglobin, B-type natriuretic peptide, and inflammatory cells/molecules values (p < 0.05). SGLT2-I users vs. Non-SGLT2-I users, as evaluated by OCT, evidenced the highest values of minimum FCT, and lowest values of lipid arc degree and macrophage grade (p < 0.05). At the follow-up end, SGLT2-I users vs. Non-SGLT2-I users had a lower rate of MACEs [n 12 (10.8%) vs. n 57 (22.1%); p < 0.05]. Finally, Hb1Ac values (1.930, [CI 95%: 1.149-2.176]), macrophage grade (1.188, [CI 95%: 1.073-1.315]), and SGLT2-I therapy (0.342, [CI 95%: 0.180-0.651]) were independent predictors of MACEs at 1 year of follow-up.
Conclusions: SGLT2-I therapy may reduce about 65% the risk to have MACEs at 1 year of follow-up, via ameliorative effects on glucose homeostasis, and by the reduction of systemic inflammatory burden, and local effects on the atherosclerotic plaque inflammation, lipids' deposit, and FCT in Mv-NOCS patients with T2DM.
Keywords: FCT; Inflammatory burden; MACEs; Mv-NOCS; SGLT2-I.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134(10):752–772. doi: 10.1161/CIRCULATIONAHA.116.021887. - DOI - PubMed
-
- Sardu C, Massetti M, Testa N, Martino LD, Castellano G, Turriziani F, Sasso FC, Torella M, De Feo M, Santulli G, Paolisso G, Marfella R. Effects of sodium–glucose transporter 2 inhibitors (SGLT2-I) in patients with ischemic heart disease (IHD) treated by coronary artery bypass grafting via MiECC: inflammatory burden, and clinical outcomes at 5 years of follow-up. Front Pharmacol. 2021;12:777083. doi: 10.3389/fphar.2021.777083. - DOI - PMC - PubMed
-
- Paolisso P, Bergamaschi L, Santulli G, Gallinoro E, Cesaro A, Gragnano F, Sardu C, Mileva N, Foà A, Armillotta M, Sansonetti A, Amicone S, Impellizzeri A, Casella G, Mauro C, Vassilev D, Marfella R, Calabrò P, Barbato E, Pizzi C. Infarct size, inflammatory burden, and admission hyperglycemia in diabetic patients with acute myocardial infarction treated with SGLT2-inhibitors: a multicenter international registry. Cardiovasc Diabetol. 2022;21(1):77. doi: 10.1186/s12933-022-01506-8. - DOI - PMC - PubMed
-
- Marfella R, Sardu C, Balestrieri ML, Siniscalchi M, Minicucci F, Signoriello G, Calabrò P, Mauro C, Pieretti G, Coppola A, Nicoletti G, Rizzo MR, Paolisso G, Barbieri M. Effects of incretin treatment on cardiovascular outcomes in diabetic STEMI-patients with culprit obstructive and multivessel non obstructive-coronary-stenosis. Diabetol Metab Syndr. 2018;10:1. doi: 10.1186/s13098-017-0304-3. - DOI - PMC - PubMed
-
- Milzi A, Burgmaier M, Burgmaier K, et al. Type 2 diabetes mellitus is associated with a lower fibrous cap thickness but has no impact on calcification morphology: an intracoronary optical coherence tomography study. Cardiovasc Diabetol. 2017;16:152. doi: 10.1186/s12933-017-0635-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

