Cerebrospinal fluid lipoproteins inhibit α-synuclein aggregation by interacting with oligomeric species in seed amplification assays
- PMID: 37005644
- PMCID: PMC10068178
- DOI: 10.1186/s13024-023-00613-8
Cerebrospinal fluid lipoproteins inhibit α-synuclein aggregation by interacting with oligomeric species in seed amplification assays
Abstract
Background: Aggregation of α-synuclein (α-syn) is a prominent feature of Parkinson's disease (PD) and other synucleinopathies. Currently, α-syn seed amplification assays (SAAs) using cerebrospinal fluid (CSF) represent the most promising diagnostic tools for synucleinopathies. However, CSF itself contains several compounds that can modulate the aggregation of α-syn in a patient-dependent manner, potentially undermining unoptimized α-syn SAAs and preventing seed quantification.
Methods: In this study, we characterized the inhibitory effect of CSF milieu on detection of α-syn aggregates by means of CSF fractionation, mass spectrometry, immunoassays, transmission electron microscopy, solution nuclear magnetic resonance spectroscopy, a highly accurate and standardized diagnostic SAA, and different in vitro aggregation conditions to evaluate spontaneous aggregation of α-syn.
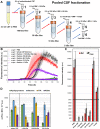
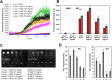
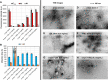
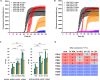
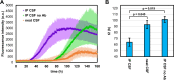
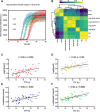
Results: We found the high-molecular weight fraction of CSF (> 100,000 Da) to be highly inhibitory on α-syn aggregation and identified lipoproteins to be the main drivers of this effect. Direct interaction between lipoproteins and monomeric α-syn was not detected by solution nuclear magnetic resonance spectroscopy, on the other hand we observed lipoprotein-α-syn complexes by transmission electron microscopy. These observations are compatible with hypothesizing an interaction between lipoproteins and oligomeric/proto-fibrillary α-syn intermediates. We observed significantly slower amplification of α-syn seeds in PD CSF when lipoproteins were added to the reaction mix of diagnostic SAA. Additionally, we observed a decreased inhibition capacity of CSF on α-syn aggregation after immunodepleting ApoA1 and ApoE. Finally, we observed that CSF ApoA1 and ApoE levels significantly correlated with SAA kinetic parameters in n = 31 SAA-negative control CSF samples spiked with preformed α-syn aggregates.
Conclusions: Our results describe a novel interaction between lipoproteins and α-syn aggregates that inhibits the formation of α-syn fibrils and could have relevant implications. Indeed, the donor-specific inhibition of CSF on α-syn aggregation explains the lack of quantitative results from analysis of SAA-derived kinetic parameters to date. Furthermore, our data show that lipoproteins are the main inhibitory components of CSF, suggesting that lipoprotein concentration measurements could be incorporated into data analysis models to eliminate the confounding effects of CSF milieu on α-syn quantification efforts.
Keywords: Cerebrospinal fluid; Lipoproteins; RT-QuIC; Seed amplification assays; α-synuclein.
© 2023. The Author(s).
Conflict of interest statement
The authors declare the following competing financial interest(s): Prof. Parnetti served as Member of Advisory Boards for Fujirebio, IBL, Roche and Merck. Dr. Concha, Ms. Farris, and Mr. Ma are inventors on several patents related to SAA technology (PMCA) and are associated to Amprion Inc., a biotech company focused on the commercial utilization of SAA for diagnosis. All the other authors declare no financial and non-financial competing interests.
Figures




References
-
- Paciotti S, Bellomo G, Gatticchi L, Parnetti L. Are We Ready for Detecting α-Synuclein Prone to Aggregation in Patients? The Case of “Protein-Misfolding Cyclic Amplification” and “Real-Time Quaking-Induced Conversion” as Diagnostic Tools. Front Neurol. 2018;9:415. doi: 10.3389/fneur.2018.00415. - DOI - PMC - PubMed
-
- Iranzo A, Fairfoul G, Ayudhaya ACN, Serradell M, Gelpi E, Vilaseca I, et al. Detection of α-synuclein in CSF by RT-QuIC in patients with isolated rapid-eye-movement sleep behaviour disorder: a longitudinal observational study. Lancet Neurol. 2021;20:203–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

