Low-dose methotrexate-induced renal failure in a patient with ectopic pregnancy: a case report
- PMID: 37005654
- PMCID: PMC10068185
- DOI: 10.1186/s13256-023-03834-z
Low-dose methotrexate-induced renal failure in a patient with ectopic pregnancy: a case report
Abstract
Background: Methotrexate is an anticancer drug from the antimetabolite class. It is also used in gynecology and obstetrics for the medical treatment of ectopic pregnancies. Low-dose methotrexate-induced adverse toxic effects are rare. We report a case of toxic effect associated with severe renal insufficiency induced by LD-MTX (Low-Dose Methotrexate) for ectopic pregnancy.
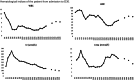
Case presentation: A 46-year-old Chinese woman was in an operation for an ectopic pregnancy of tubal interstitial pregnancy. The embryo villus was so little that we were not sure if it was evacuated, then it was followed with 50 mg methotrexate injection of intramuscular adjacent the uterine horn in the operation. 48 hour later after injection the patient presented with renal failure. The personalized genetic testing showed that MTHFR (677C > T) and ABCB1 (3435T > C) were detected. Gradually, the symptoms improved after calcium leucovorin (CF) rescue, continuous renal replacement therapy (CRRT), promoting blood system regeneration, and multiple supportive treatments.
Conclusions: When toxic effects are suspected, detecting the polymorphisms of an MTHFR gene and monitoring MTX concentration in blood could assist us to formulate individualized and active treatments. The management should be multidisciplinary and as much as possible within an intensive care unit.
Keywords: Ectopic pregnancy; Methotrexate; Renal failure; Toxicity.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Severe Adverse Toxic Effects of Low-Dose Methotrexate Treatment on an Ectopic Pregnancy Patient With Methylenetetrahydrofolate Reductase Mutations: A Case Report.Front Med (Lausanne). 2021 Nov 18;8:738315. doi: 10.3389/fmed.2021.738315. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34869432 Free PMC article.
-
The effect of parental 5,10-methylenetetrahydrofolate reductase 677C/T and 1298A/C gene polymorphisms on response to single-dose methotrexate in tubal ectopic pregnancy.J Matern Fetal Neonatal Med. 2017 May;30(10):1232-1237. doi: 10.1080/14767058.2016.1209652. Epub 2016 Aug 2. J Matern Fetal Neonatal Med. 2017. PMID: 27379466
-
Overview and guidelines of off-label use of methotrexate in ectopic pregnancy: report by CNGOF.Eur J Obstet Gynecol Reprod Biol. 2016 Oct;205:105-9. doi: 10.1016/j.ejogrb.2016.07.489. Epub 2016 Aug 3. Eur J Obstet Gynecol Reprod Biol. 2016. PMID: 27572300 Review.
-
Methotrexate-induced toxidermia and pancytopenia in a patient with ectopic pregnancy: a case report.J Med Case Rep. 2021 Dec 7;15(1):579. doi: 10.1186/s13256-021-03111-x. J Med Case Rep. 2021. PMID: 34872594 Free PMC article.
-
[Evidence-based evaluation and expertise of methotrexate off label use in gynaecology and obstetrics: work of the CNGOF].J Gynecol Obstet Biol Reprod (Paris). 2015 Mar;44(3):230-6. doi: 10.1016/j.jgyn.2014.12.008. Epub 2015 Feb 7. J Gynecol Obstet Biol Reprod (Paris). 2015. PMID: 25661495 Review. French.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials