The changing landscape of drug clinical trials on cardiometabolic diseases in China, 2009-2021
- PMID: 37005689
- PMCID: PMC10067219
- DOI: 10.1186/s13098-023-01043-8
The changing landscape of drug clinical trials on cardiometabolic diseases in China, 2009-2021
Abstract
Background: Cardiometabolic disease is a clinical syndrome characterized by multiple metabolic disorders, with atherosclerosis as the core and cardiovascular and cerebrovascular events as the outcome. Drug research and development (R&D) in cardiometabolic diseases has grown rapidly worldwide. However, the development of cardiometabolic drug clinical trials in China remains unclear. This study aims to depict the changing landscape of drug clinical trials for cardiometabolic diseases in China during 2009-2021.
Methods: The detailed information of drug trials on cardiometabolic diseases registered in the National Medical Products Administration (NMPA) Registration and Information Disclosure Platform was collected between January 1, 2009, and July 1, 2021. The landscape of cardiometabolic drug clinical trials was analyzed by the characteristics, time trends, indications, pharmacological mechanisms, and geographical distribution.
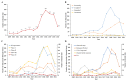
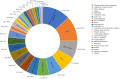
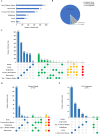
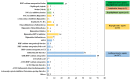
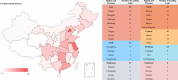
Results: A total of 2466 drug clinical trials on cardiometabolic diseases were extracted and analyzed. The annual number of drug trials increased rapidly in the past twelve years. Among all the trials, the bioequivalence trials (1428; 58.3%) accounted for the largest proportion, followed by phase I (555; 22.5%), phase III (278; 11.3%), phase II (169; 6.9%), and phase IV (26; 1.1%). Of 2466 trials, 2133 (86.5%) trials were monomer drugs, only 236 (9.6%) trials were polypills and 97 (3.9%) were traditional Chinese medicine (TCM) compounds. In terms of pharmacological mechanisms, the number of trials in dihydropyridine (DHP) calcium antagonists 321 (11.9%) ranked first, while trials in angiotensin receptor blocker (ARB) 289 (10.7%) and dipeptidyl peptidase-4 (DPP-4) inhibitor 205 (7.6%) ranked second and third place respectively. Of 236 chemical polypills trials, 23 (9.7%) polypills were the combination of DHP calcium antagonists and statins, while others were the combination of two same pharmacological effect agents. As for the geographical distribution of leading units, 36 trials were led by principal investigators (PI) units from Beijing, followed by Jiangsu (n = 29), Shanghai (n = 19), Guangdong (n = 19), and Hunan (n = 19), showing an uneven regional distribution.
Conclusions: Great progress has been made in drug clinical trials on cardiometabolic diseases, especially in antihypertensive agents, hypoglycemic agents, and hypolipidemic agents. However, the insufficient innovation of first-in-class drugs and polypills should be carefully considered by all stakeholders in drug trials.
Keywords: Cardiometabolic disease; Changing landscape; China; Drug clinical trial.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- National Center for Cardiovascular Disease C. Annual Report on Cardiovascular Health and Disease in China(2020) Beijing: Science Press; 2021.
-
- Saxon DR, Reiter-Brennan C, Blaha MJ, Eckel RH. Cardiometabolic Medicine: Development of a New Subspecialty.J Clin Endocrinol Metab.2020;105(7). - PubMed
Grants and funding
- Z211100002121063/Beijing Nova Program from Beijing Municipal Science & Technology Commission
- Z201100006820002/Beijing Nova Program from Beijing Municipal Science & Technology Commission
- 2020YFC2004705/National Key R&D Program of China
- 2021-I2M-5-003/CAMS Innovation Fund for Medical Sciences
- 81825003, 91957123, 81800327, 81900272, 82270376/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

