Varicella Zoster Virus infects mucosal associated Invariant T cells
- PMID: 37006246
- PMCID: PMC10063790
- DOI: 10.3389/fimmu.2023.1121714
Varicella Zoster Virus infects mucosal associated Invariant T cells
Abstract
Introduction: Mucosal Associated Invariant T (MAIT) cells are innate-like T cells that respond to conserved pathogen-derived vitamin B metabolites presented by the MHC class I related-1 molecule (MR1) antigen presentation pathway. Whilst viruses do not synthesize these metabolites, we have reported that varicella zoster virus (VZV) profoundly suppresses MR1 expression, implicating this virus in manipulation of the MR1:MAIT cell axis. During primary infection, the lymphotropism of VZV is likely to be instrumental in hematogenous dissemination of virus to gain access to cutaneous sites where it clinically manifests as varicella (chickenpox). However, MAIT cells, which are found in the blood and at mucosal and other organ sites, have yet to be examined in the context of VZV infection. The goal of this study was to examine any direct impact of VZV on MAIT cells.
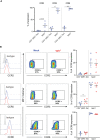
Methods: Using flow cytometry, we interrogated whether primary blood derived MAIT cells are permissive to infection by VZV whilst further analysing differential levels of infection between various MAIT cell subpopulations. Changes in cell surface extravasation, skin homing, activation and proliferation markers after VZV infection of MAIT cells was also assessed via flow cytometry. Finally the capacity of MAIT cells to transfer infectious virus was tested through an infectious center assay and imaged via fluorescence microscopy.
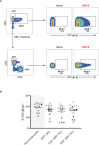
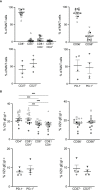
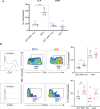
Results: We identify primary blood-derived MAIT cells as being permissive to VZV infection. A consequence of VZV infection of MAIT cells was their capacity to transfer infectious virus to other permissive cells, consistent with MAIT cells supporting productive infection. When subgrouping MAIT cells by their co- expression of a variety cell surface markers, there was a higher proportion of VZV infected MAIT cells co-expressing CD4+ and CD4+/CD8+ MAIT cells compared to the more phenotypically dominant CD8+ MAIT cells, whereas infection was not associated with differences in co-expression of CD56 (MAIT cell subset with enhanced responsiveness to innate cytokine stimulation), CD27 (co-stimulatory) or PD-1 (immune checkpoint). Infected MAIT cells retained high expression of CCR2, CCR5, CCR6, CLA and CCR4, indicating a potentially intact capacity for transendothelial migration, extravasation and trafficking to skin sites. Infected MAIT cells also displayed increased expression of CD69 (early activation) and CD71 (proliferation) markers.
Discussion: These data identify MAIT cells as being permissive to VZV infection and identify impacts of such infection on co- expressed functional markers.
Keywords: MAIT cells; Varicella Zoster Virus; herpesvirus; innate-like T cells; productive infection.
Copyright © 2023 Purohit, Corbett, Slobedman and Abendroth.
Conflict of interest statement
AC is an inventor on patents describing MR1-tetramers. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer LH declared a shared affiliation with the author AC to the handling editor at time of review.
Figures

Similar articles
-
Varicella Zoster Virus disrupts MAIT cell polyfunctional effector responses.PLoS Pathog. 2024 Aug 7;20(8):e1012372. doi: 10.1371/journal.ppat.1012372. eCollection 2024 Aug. PLoS Pathog. 2024. PMID: 39110717 Free PMC article.
-
Varicella Zoster Virus Impairs Expression of the Nonclassical Major Histocompatibility Complex Class I-Related Gene Protein (MR1).J Infect Dis. 2023 Feb 1;227(3):391-401. doi: 10.1093/infdis/jiab526. J Infect Dis. 2023. PMID: 34648018 Free PMC article.
-
Tropism of varicella-zoster virus for human tonsillar CD4(+) T lymphocytes that express activation, memory, and skin homing markers.J Virol. 2002 Nov;76(22):11425-33. doi: 10.1128/jvi.76.22.11425-11433.2002. J Virol. 2002. PMID: 12388703 Free PMC article.
-
MHC class I-related molecule, MR1, and mucosal-associated invariant T cells.Immunol Rev. 2016 Jul;272(1):120-38. doi: 10.1111/imr.12423. Immunol Rev. 2016. PMID: 27319347 Review.
-
Viral Impacts on MR1 Antigen Presentation to MAIT Cells.Crit Rev Immunol. 2021;41(5):49-67. doi: 10.1615/CritRevImmunol.2022041981. Crit Rev Immunol. 2021. PMID: 35381139 Review.
Cited by
-
Transcriptional and functional remodeling of lung-resident T cells and macrophages by Simian varicella virus infection.Front Immunol. 2024 Jun 3;15:1408212. doi: 10.3389/fimmu.2024.1408212. eCollection 2024. Front Immunol. 2024. PMID: 38887303 Free PMC article.
-
Herpes simplex virus type 1 impairs mucosal-associated invariant T cells.mBio. 2025 May 14;16(5):e0388724. doi: 10.1128/mbio.03887-24. Epub 2025 Mar 26. mBio. 2025. PMID: 40135871 Free PMC article.
-
Varicella Zoster Virus disrupts MAIT cell polyfunctional effector responses.PLoS Pathog. 2024 Aug 7;20(8):e1012372. doi: 10.1371/journal.ppat.1012372. eCollection 2024 Aug. PLoS Pathog. 2024. PMID: 39110717 Free PMC article.
-
Varicella Zoster Virus Downregulates Expression of the Nonclassical Antigen Presentation Molecule CD1d.J Infect Dis. 2024 Aug 16;230(2):e416-e426. doi: 10.1093/infdis/jiad512. J Infect Dis. 2024. PMID: 37972257 Free PMC article.
References
-
- Tilloy F, Treiner E, Park S-H, Garcia C, Lemonnier F, de la Salle H, et al. . An invariant T cell receptor α chain defines a novel TAP-independent major histocompatibility complex class ib–restricted α/β T cell subpopulation in mammals. J Exp Med (1999) 189(12):1907–21. doi: 10.1084/jem.189.12.1907 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

