Heterologous prime-boost immunisation with mRNA- and AdC68-based 2019-nCoV variant vaccines induces broad-spectrum immune responses in mice
- PMID: 37006275
- PMCID: PMC10050358
- DOI: 10.3389/fimmu.2023.1142394
Heterologous prime-boost immunisation with mRNA- and AdC68-based 2019-nCoV variant vaccines induces broad-spectrum immune responses in mice
Abstract
The ongoing evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 or 2019-nCoV) variants has been associated with the transmission and pathogenicity of COVID-19. Therefore, exploring the optimal immunisation strategy to improve the broad-spectrum cross-protection ability of COVID-19 vaccines is of great significance. Herein, we assessed different heterologous prime-boost strategies with chimpanzee adenovirus vector-based COVID-19 vaccines plus Wuhan-Hu-1 (WH-1) strain (AdW) and Beta variant (AdB) and mRNA-based COVID-19 vaccines plus WH-1 strain (ARW) and Omicron (B.1.1.529) variant (ARO) in 6-week-old female BALB/c mice. AdW and AdB were administered intramuscularly or intranasally, while ARW and ARO were administered intramuscularly. Intranasal or intramuscular vaccination with AdB followed by ARO booster exhibited the highest levels of cross-reactive IgG, pseudovirus-neutralising antibody (PNAb) responses, and angiotensin-converting enzyme-2 (ACE2)-binding inhibition rates against different 2019-nCoV variants among all vaccination groups. Moreover, intranasal AdB vaccination followed by ARO induced higher levels of IgA and neutralising antibody responses against live 2019-nCoV than intramuscular AdB vaccination followed by ARO. A single dose of AdB administered intranasally or intramuscularly induced broader cross-NAb responses than AdW. Th1-biased cellular immune response was induced in all vaccination groups. Intramuscular vaccination-only groups exhibited higher levels of Th1 cytokines than intranasal vaccination-only and intranasal vaccination-containing groups. However, no obvious differences were found in the levels of Th2 cytokines between the control and all vaccination groups. Our findings provide a basis for exploring vaccination strategies against different 2019-nCoV variants to achieve high broad-spectrum immune efficacy.
Keywords: ARCoV; COVID-19; ChAdTS-S; SARS-COV-2 variants; heterologous prime-boost; intramuscular; intranasal.
Copyright © 2023 Li, Liu, Li, Peng, Li, Ying, Zhang, Liu, Wu, Zhao, Yang, Cao, Huang, Shi, Xu, Wang, Yue, Suo, Nie, Huang, Li and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

 , vaccination;
, vaccination;  , bleeding;
, bleeding;  , spleen lymphocyte isolation. Im, intramuscular vaccination; in, intranasal vaccination.
, spleen lymphocyte isolation. Im, intramuscular vaccination; in, intranasal vaccination.



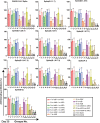

Similar articles
-
Immunogenicity of an adenovirus-vectored bivalent vaccine against wild type SARS-CoV-2 and Omicron variants in a murine model.Vaccine. 2024 Feb 27;42(6):1292-1299. doi: 10.1016/j.vaccine.2024.01.073. Epub 2024 Jan 30. Vaccine. 2024. PMID: 38296705
-
Combining intramuscular and intranasal homologous prime-boost with a chimpanzee adenovirus-based COVID-19 vaccine elicits potent humoral and cellular immune responses in mice.Emerg Microbes Infect. 2022 Dec;11(1):1890-1899. doi: 10.1080/22221751.2022.2097479. Emerg Microbes Infect. 2022. PMID: 35775819 Free PMC article.
-
Heterologous ChAdOx1 nCoV-19 and BNT162b2 prime-boost vaccination elicits potent neutralizing antibody responses and T cell reactivity against prevalent SARS-CoV-2 variants.EBioMedicine. 2022 Jan;75:103761. doi: 10.1016/j.ebiom.2021.103761. Epub 2021 Dec 17. EBioMedicine. 2022. PMID: 34929493 Free PMC article. Clinical Trial.
-
SARS-CoV-2 S1 Subunit Booster Vaccination Elicits Robust Humoral Immune Responses in Aged Mice.Microbiol Spectr. 2023 Jun 15;11(3):e0436322. doi: 10.1128/spectrum.04363-22. Epub 2023 May 10. Microbiol Spectr. 2023. PMID: 37162333 Free PMC article.
-
Chronovaccination: Harnessing circadian rhythms to optimize immunisation strategies.Front Immunol. 2022 Oct 7;13:977525. doi: 10.3389/fimmu.2022.977525. eCollection 2022. Front Immunol. 2022. PMID: 36275731 Free PMC article. Review.
References
-
- Aleem A, Akbar Samad AB, Slenker AK. Emerging variants of SARS-CoV-2 and novel therapeutics against coronavirus (COVID-19). In: StatPearls. Treasure Island (FL: StatPearls Publishing; (2022). - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

