Adrenergic receptor signaling regulates the CD40-receptor mediated anti-tumor immunity
- PMID: 37006295
- PMCID: PMC10050348
- DOI: 10.3389/fimmu.2023.1141712
Adrenergic receptor signaling regulates the CD40-receptor mediated anti-tumor immunity
Abstract
Inroduction: Anti-CD40 agonistic antibody (αCD40), an activator of dendritic cells (DC) can enhance antigen presentation and activate cytotoxic T-cells against poorly immunogenic tumors. However, cancer immunotherapy trials also suggest that αCD40 is only moderately effective in patients, falling short of achieving clinical success. Identifying factors that decrease αCD40 immune-stimulating effects can aid the translation of this agent to clinical reality.
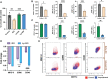
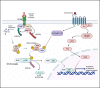
Method/results: Here, we reveal that β-adrenergic signaling on DCs directly interferes with αCD40 efficacy in immunologically cold head and neck tumor model. We discovered that β-2 adrenergic receptor (β2AR) activation rewires CD40 signaling in DCs by directly inhibiting the phosphorylation of IκBα and indirectly by upregulating levels of phosphorylated-cAMP response element-binding protein (pCREB). Importantly, the addition of propranolol, a pan β-Blocker reprograms the CD40 pathways, inducing superior tumor regressions, increased infiltration of cytotoxic T-cells, and a reduced burden of regulatory T-cells in tumors compared to monotherapy.
Discussion/conclusion: Thus, our study highlights an important mechanistic link between stress-induced β2AR signaling and reduced αCD40 efficacy in cold tumors, providing a new combinatorial approach to improve clinical outcomes in patients.
Keywords: Anti-CD40 agonist antibody; adrenergic signaling; anti-tumor immunity; immunotherapy; propranalol.
Copyright © 2023 Singh and Ranjan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Fusion of Bacterial Flagellin to a Dendritic Cell-Targeting αCD40 Antibody Construct Coupled With Viral or Leukemia-Specific Antigens Enhances Dendritic Cell Maturation and Activates Peptide-Responsive T Cells.Front Immunol. 2020 Nov 12;11:602802. doi: 10.3389/fimmu.2020.602802. eCollection 2020. Front Immunol. 2020. PMID: 33281829 Free PMC article.
-
Efficacy of CD40 Agonists Is Mediated by Distinct cDC Subsets and Subverted by Suppressive Macrophages.Cancer Res. 2022 Oct 17;82(20):3785-3801. doi: 10.1158/0008-5472.CAN-22-0094. Cancer Res. 2022. PMID: 35979635 Free PMC article.
-
Sufficiency of CD40 activation and immune checkpoint blockade for T cell priming and tumor immunity.Proc Natl Acad Sci U S A. 2020 Apr 7;117(14):8022-8031. doi: 10.1073/pnas.1918971117. Epub 2020 Mar 25. Proc Natl Acad Sci U S A. 2020. PMID: 32213589 Free PMC article.
-
A potential novel cancer immunotherapy: Agonistic anti-CD40 antibodies.Drug Discov Today. 2024 Mar;29(3):103893. doi: 10.1016/j.drudis.2024.103893. Epub 2024 Jan 23. Drug Discov Today. 2024. PMID: 38272173 Review.
-
Dendritic cell gene therapy.Surg Oncol Clin N Am. 2002 Jul;11(3):645-60. doi: 10.1016/s1055-3207(02)00027-3. Surg Oncol Clin N Am. 2002. PMID: 12487060 Review.
Cited by
-
Neuroscience in peripheral cancers: tumors hijacking nerves and neuroimmune crosstalk.MedComm (2020). 2024 Oct 31;5(11):e784. doi: 10.1002/mco2.784. eCollection 2024 Nov. MedComm (2020). 2024. PMID: 39492832 Free PMC article. Review.
-
Investigating the crosstalk between chronic stress and immune cells: implications for enhanced cancer therapy.Front Neurosci. 2023 Nov 28;17:1321176. doi: 10.3389/fnins.2023.1321176. eCollection 2023. Front Neurosci. 2023. PMID: 38089966 Free PMC article. Review.
-
Advances on Delivery System of Active Ingredients of Dried Toad Skin and Toad Venom.Int J Nanomedicine. 2024 Jul 18;19:7273-7305. doi: 10.2147/IJN.S469742. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39050871 Free PMC article. Review.
-
The β2-adrenergic biased agonist nebivolol inhibits the development of Th17 and the response of memory Th17 cells in an NF-κB-dependent manner.Front Immunol. 2024 Oct 9;15:1446424. doi: 10.3389/fimmu.2024.1446424. eCollection 2024. Front Immunol. 2024. PMID: 39445009 Free PMC article.
-
The Neuroimmune Axis and Its Therapeutic Potential for Primary Liver Cancer.Int J Mol Sci. 2024 Jun 5;25(11):6237. doi: 10.3390/ijms25116237. Int J Mol Sci. 2024. PMID: 38892423 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

