Do peripheral neuropathies differ among immune checkpoint inhibitors? Reports from the European post-marketing surveillance database in the past 10 years
- PMID: 37006303
- PMCID: PMC10060793
- DOI: 10.3389/fimmu.2023.1134436
Do peripheral neuropathies differ among immune checkpoint inhibitors? Reports from the European post-marketing surveillance database in the past 10 years
Abstract
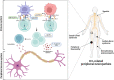
Although the immunotherapy advent has revolutionized cancer treatment, it, unfortunately, does not spare cancer patients from possible immune-related adverse events (irAEs), which can also involve the peripheral nervous system. Immune checkpoint inhibitors (ICIs), blocking cytotoxic T-lymphocyteassociated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), or programmed cell death ligand 1 (PD-L1), can induce an immune imbalance and cause different peripheral neuropathies (PNs). Considering the wide range of PNs and their high impact on the safety and quality of life for cancer patients and the availability of large post-marketing surveillance databases, we chose to analyze the characteristics of ICI-related PNs reported as suspected drug reactions from 2010 to 2020 in the European real-world context. We analyzed data collected in the European pharmacovigilance database, Eudravigilance, and conducted a systematic and disproportionality analysis. In our study, we found 735 reports describing 766 PNs occurred in patients treated with ICIs. These PNs included Guillain-Barré syndrome, Miller-Fisher syndrome, neuritis, and chronic inflammatory demyelinating polyradiculoneuropathy. These ADRs were often serious, resulting in patient disability or hospitalization. Moreover, our disproportionality analysis revealed an increased reporting frequency of PNs with tezolizumab compared to other ICIs. Guillain-Barré syndrome is a notable potential PN related to ICIs, as it is associated with a significant impact on patient safety and has had unfavorable outcomes, including a fatal one. Continued monitoring of the safety profile of ICIs in real-life settings is necessary, especially considering the increased frequency of PNs associated with atezolizumab compared with other ICIs.
Keywords: immune checkpoint inhibitors; immune-related adverse events; immunotherapy; neurological toxicity; peripheral neuropathies; post-marketing surveillance; translational research.
Copyright © 2023 Ruggiero, Balzano, Di Napoli, Fraenza, Pentella, Riccardi, Donniacuo, Tesorone, Danesi, Del Re, Rossi and Capuano.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

