The role of NOD-like receptors in innate immunity
- PMID: 37006312
- PMCID: PMC10050748
- DOI: 10.3389/fimmu.2023.1122586
The role of NOD-like receptors in innate immunity
Abstract
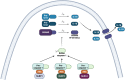
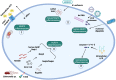
The innate immune system in vertebrates and invertebrates relies on conserved receptors and ligands, and pathways that can rapidly initiate the host response against microbial infection and other sources of stress and danger. Research into the family of NOD-like receptors (NLRs) has blossomed over the past two decades, with much being learned about the ligands and conditions that stimulate the NLRs and the outcomes of NLR activation in cells and animals. The NLRs play key roles in diverse functions, ranging from transcription of MHC molecules to initiation of inflammation. Some NLRs are activated directly by their ligands, while other ligands may have indirect effects on the NLRs. New findings in coming years will undoubtedly shed more light on molecular details involved in NLR activation, as well as the physiological and immunological outcomes of NLR ligation.
Keywords: inflammasome; inflammation; innate immunity; nod-like receptors; pathogen recognition receptors; pathogen-associated molecular patterns; toll-like receptors.
Copyright © 2023 Almeida-da-Silva, Savio, Coutinho-Silva and Ojcius.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

