Giardia duodenalis-induced G0/G1 intestinal epithelial cell cycle arrest and apoptosis involve activation of endoplasmic reticulum stress in vitro
- PMID: 37006313
- PMCID: PMC10050679
- DOI: 10.3389/fimmu.2023.1127552
Giardia duodenalis-induced G0/G1 intestinal epithelial cell cycle arrest and apoptosis involve activation of endoplasmic reticulum stress in vitro
Abstract
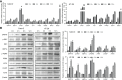
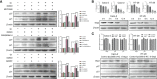
Giardia duodenalis is a zoonotic intestinal protozoan parasite that may cause host diarrhea and chronic gastroenteritis, resulting in great economic losses annually and representing a significant public health burden across the world. However, thus far, our knowledge on the pathogenesis of Giardia and the related host cell responses is still extensively limited. The aim of this study is to assess the role of endoplasmic reticulum (ER) stress in regulating G0/G1 cell cycle arrest and apoptosis during in vitro infection of intestinal epithelial cells (IECs) with Giardia. The results showed that the mRNA levels of ER chaperone proteins and ER-associated degradation genes were increased and the expression levels of the main unfolded protein response (UPR)-related proteins (GRP78, p-PERK, ATF4, CHOP, p-IRE1, XBP1s and ATF6) were increased upon Giardia exposure. In addition, cell cycle arrest was determined to be induced by UPR signaling pathways (IRE1, PERK and ATF6) through upregulation of p21 and p27 levels and promotion of E2F1-RB complex formation. Upregulation of p21 and p27 expression was shown to be related to Ufd1-Skp2 signaling. Therefore, the cell cycle arrest was induced by ER stress when infected with Giardia. Furthermore, the apoptosis of the host cell was also assessed after exposure to Giardia. The results indicated that apoptosis would be promoted by UPR signaling (PERK and ATF6), but would be suppressed by the hyperphosphorylation of AKT and hypophosphorylation of JNK that were modulated by IRE1 pathway. Taken together, both of the cell cycle arrest and apoptosis of IECs induced by Giardia exposure involved the activation of the UPR signaling. The findings of this study will deepen our understanding of the pathogenesis of Giardia and the associated regulatory network.
Keywords: ER stress; Giardia duodenalis; IECs; UPR; apoptosis; cell cycle arrest.
Copyright © 2023 Yu, Zhao, Qin, Li, Guo and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

