Glycosaminoglycans: What Remains To Be Deciphered?
- PMID: 37006755
- PMCID: PMC10052243
- DOI: 10.1021/jacsau.2c00569
Glycosaminoglycans: What Remains To Be Deciphered?
Abstract
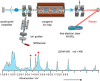
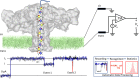
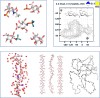
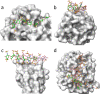
Glycosaminoglycans (GAGs) are complex polysaccharides exhibiting a vast structural diversity and fulfilling various functions mediated by thousands of interactions in the extracellular matrix, at the cell surface, and within the cells where they have been detected in the nucleus. It is known that the chemical groups attached to GAGs and GAG conformations comprise "glycocodes" that are not yet fully deciphered. The molecular context also matters for GAG structures and functions, and the influence of the structure and functions of the proteoglycan core proteins on sulfated GAGs and vice versa warrants further investigation. The lack of dedicated bioinformatic tools for mining GAG data sets contributes to a partial characterization of the structural and functional landscape and interactions of GAGs. These pending issues will benefit from the development of new approaches reviewed here, namely (i) the synthesis of GAG oligosaccharides to build large and diverse GAG libraries, (ii) GAG analysis and sequencing by mass spectrometry (e.g., ion mobility-mass spectrometry), gas-phase infrared spectroscopy, recognition tunnelling nanopores, and molecular modeling to identify bioactive GAG sequences, biophysical methods to investigate binding interfaces, and to expand our knowledge and understanding of glycocodes governing GAG molecular recognition, and (iii) artificial intelligence for in-depth investigation of GAGomic data sets and their integration with proteomics.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Varki A.; Cummings R. D.; Aebi M.; Packer N. H.; Seeberger P. H.; Esko J. D.; Stanley P.; Hart G.; Darvill A.; Kinoshita T.; Prestegard J. J.; Schnaar R. L.; Freeze H. H.; Marth J. D.; Bertozzi C. R.; Etzler M. E.; Frank M.; Vliegenthart J. F.; Lutteke T.; Perez S.; Bolton E.; Rudd P.; Paulson J.; Kanehisa M.; Toukach P.; Aoki-Kinoshita K. F.; Dell A.; Narimatsu H.; York W.; Taniguchi N.; Kornfeld S. Symbol Nomenclature for Graphical Representations of Glycans. Glycobiology 2015, 25 (12), 1323–4. 10.1093/glycob/cwv091. - DOI - PMC - PubMed
