ACSM3 suppresses proliferation and induces apoptosis and cell cycle arrest in acute myeloid leukemia cells via the regulation of IGF2BP2
- PMID: 37006876
- PMCID: PMC10061044
- DOI: 10.3892/etm.2023.11876
ACSM3 suppresses proliferation and induces apoptosis and cell cycle arrest in acute myeloid leukemia cells via the regulation of IGF2BP2
Erratum in
-
Erratum: [Corrigendum] ACSM3 suppresses proliferation and induces apoptosis and cell cycle arrest in acute myeloid leukemia cells via the regulation of IGF2BP2.Exp Ther Med. 2024 Jul 25;28(4):374. doi: 10.3892/etm.2024.12663. eCollection 2024 Oct. Exp Ther Med. 2024. PMID: 39091633 Free PMC article.
Abstract
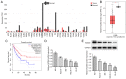
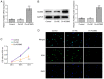
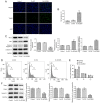
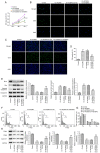
Acyl-CoA medium-chain synthetase-3 (ACSM3) has been reported to be involved in the malignant progression of multiple types of human cancer. Nevertheless, the role of ACSM3 in acute myeloid leukemia (AML) and its exact mechanism of action are as yet undefined. In the present study, the expression levels of ACSM3 and IGF2 mRNA-binding protein 2 (IGF2BP2) were evaluated using the Gene Expression Profiling Interactive Analysis database and AML cells. The Cell Counting Kit-8 assay and 5-ethynyl-2'-deoxyuridine staining were employed for the estimation of the cell proliferative activity. Induction of apoptosis and the assessment of the cell cycle were measured using flow cytometry and western blotting, respectively. The interaction of ACSM3 with IGF2BP2 was confirmed using an RNA immunoprecipitation assay. mRNA stabilization of ACSM3 following actinomycin D treatment was evaluated using reverse transcription-quantitative PCR analysis. The data indicated that the expression levels of ACSM3 were significantly downregulated, whereas those of IGF2BP2 were upregulated in tissues and AML cells. Downregulation of ACSM3 expression was closely associated with poor overall survival of patients with AML. ACSM3 overexpression repressed cell proliferative activity and induced apoptosis and cell cycle arrest. IGF2BP2 downregulated ACSM3 expression by reducing the stability of ACSM3 mRNA. In addition, IGF2BP2 overexpression counteracted the effects of ACSM3 overexpression noted on proliferation, induction of apoptosis and cell cycle arrest of HL-60 cells. In conclusion, ACSM3 repressed the cell proliferative activity and facilitated induction of apoptosis and cell cycle arrest in AML cells by modulating the expression of IGF2BP2.
Keywords: IGF2 mRNA binding protein 2; RNA-binding protein; acute myeloid leukemia; acyl-CoA medium-chain synthetase-3; apoptosis; cycle arrest.
Copyright: © Zheng et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
LINC00987 knockdown inhibits the progression of acute myeloid leukemia by suppressing IGF2BP2-mediated PA2G4 expression.Anticancer Drugs. 2022 Jan 1;33(1):e207-e217. doi: 10.1097/CAD.0000000000001188. Anticancer Drugs. 2022. PMID: 34407052
-
KLF10 upregulates ACSM3 via the PI3K/Akt signaling pathway to inhibit the malignant progression of melanoma.Oncol Lett. 2022 Jun;23(6):175. doi: 10.3892/ol.2022.13295. Epub 2022 Apr 13. Oncol Lett. 2022. PMID: 35497935 Free PMC article.
-
The Overexpression of Acyl-CoA Medium-Chain Synthetase-3 (ACSM3) Suppresses the Ovarian Cancer Progression via the Inhibition of Integrin β1/AKT Signaling Pathway.Front Oncol. 2021 Mar 31;11:644840. doi: 10.3389/fonc.2021.644840. eCollection 2021. Front Oncol. 2021. PMID: 33869039 Free PMC article.
-
Decreased RNA‑binding protein IGF2BP2 downregulates NT5DC2, which suppresses cell proliferation, and induces cell cycle arrest and apoptosis in diffuse large B‑cell lymphoma cells by regulating the p53 signaling pathway.Mol Med Rep. 2022 Sep;26(3):286. doi: 10.3892/mmr.2022.12802. Epub 2022 Jul 27. Mol Med Rep. 2022. PMID: 35894142 Free PMC article.
-
[Biological properties and sensitivity to induction therapy of differentiated cells expressing atypical immunophenotype in acute leukemia of children].Folia Med Cracov. 2001;42(3):5-80. Folia Med Cracov. 2001. PMID: 12353422 Review. Polish.
Cited by
-
A novel LGALS1-depended and immune-associated fatty acid metabolism risk model in acute myeloid leukemia stem cells.Cell Death Dis. 2024 Jul 5;15(7):482. doi: 10.1038/s41419-024-06865-6. Cell Death Dis. 2024. PMID: 38965225 Free PMC article.
-
The fatty acid-related gene signature stratifies poor prognosis patients and characterizes TIME in cutaneous melanoma.J Cancer Res Clin Oncol. 2024 Jan 27;150(2):40. doi: 10.1007/s00432-023-05580-7. J Cancer Res Clin Oncol. 2024. PMID: 38279987 Free PMC article.
-
Loosening the Lid on Shoulder Osteoarthritis: How the Transcriptome and Metabolic Syndrome Correlate with End-Stage Disease.Int J Mol Sci. 2025 Mar 28;26(7):3145. doi: 10.3390/ijms26073145. Int J Mol Sci. 2025. PMID: 40243895 Free PMC article.
-
Multifaceted roles of insulin‑like growth factor 2 mRNA binding protein 2 in human cancer (Review).Mol Med Rep. 2025 Mar;31(3):75. doi: 10.3892/mmr.2025.13441. Epub 2025 Jan 31. Mol Med Rep. 2025. PMID: 39886962 Free PMC article. Review.
References
-
- Tomizawa D, Miyamura T, Imamura T, Watanabe T, Moriya Saito A, Ogawa A, Takahashi Y, Hirayama M, Taki T, Deguchi T, et al. A risk-stratified therapy for infants with acute lymphoblastic leukemia: A report from the JPLSG MLL-10 trial. Blood. 2020;136:1813–1823. doi: 10.1182/blood.2019004741. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
