Transition-metal-catalyzed synthesis of quinazolines: A review
- PMID: 37007059
- PMCID: PMC10060649
- DOI: 10.3389/fchem.2023.1140562
Transition-metal-catalyzed synthesis of quinazolines: A review
Abstract
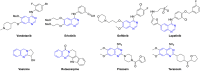
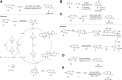
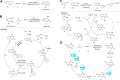
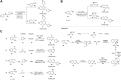
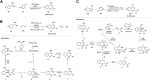
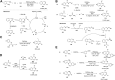
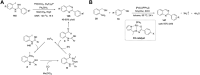
Quinazolines are a class of nitrogen-containing heterocyclic compounds with broad-spectrum of pharmacological activities. Transition-metal-catalyzed reactions have emerged as reliable and indispensable tools for the synthesis of pharmaceuticals. These reactions provide new entries into pharmaceutical ingredients of continuously increasing complexity, and catalysis with these metals has streamlined the synthesis of several marketed drugs. The last few decades have witnessed a tremendous outburst of transition-metal-catalyzed reactions for the construction of quinazoline scaffolds. In this review, the progress achieved in the synthesis of quinazolines under transition metal-catalyzed conditions are summarized and reports from 2010 to date are covered. This is presented along with the mechanistic insights of each representative methodology. The advantages, limitations, and future perspectives of synthesis of quinazolines through such reactions are also discussed.
Keywords: catalysis; mechanism; quinazoline; synthesis; transition-metal.
Copyright © 2023 Tamatam, Kim and Shin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures














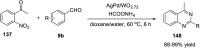

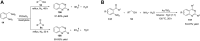
Similar articles
-
Recent advances in the transition-metal-free synthesis of quinoxalines.RSC Adv. 2021 Nov 19;11(59):37325-37353. doi: 10.1039/d1ra06942j. eCollection 2021 Nov 17. RSC Adv. 2021. PMID: 35496411 Free PMC article. Review.
-
Recent Advances in the Transition-Metal-Free Synthesis of Quinazolines.Molecules. 2023 Apr 4;28(7):3227. doi: 10.3390/molecules28073227. Molecules. 2023. PMID: 37049989 Free PMC article. Review.
-
Recent Advances in Metal-Catalyzed Approaches for the Synthesis of Quinazoline Derivatives.Molecules. 2024 May 16;29(10):2353. doi: 10.3390/molecules29102353. Molecules. 2024. PMID: 38792215 Free PMC article. Review.
-
Quinazoline and quinazolinone as important medicinal scaffolds: a comparative patent review (2011-2016).Expert Opin Ther Pat. 2018 Apr;28(4):281-297. doi: 10.1080/13543776.2018.1432596. Epub 2018 Feb 5. Expert Opin Ther Pat. 2018. PMID: 29368977 Review.
-
O-Benzoylhydroxylamines: A Versatile Electrophilic Aminating Reagent for Transition Metal-Catalyzed C-N Bond-Forming Reactions.Top Curr Chem (Cham). 2022 Dec 18;381(1):4. doi: 10.1007/s41061-022-00414-5. Top Curr Chem (Cham). 2022. PMID: 36529789 Review.
Cited by
-
Research on transition metals for the multicomponent synthesis of benzo-fused γ-lactams.RSC Adv. 2025 Jan 23;15(4):2334-2346. doi: 10.1039/d4ra08798d. eCollection 2025 Jan 23. RSC Adv. 2025. PMID: 39867320 Free PMC article. Review.
-
A comprehensive review on carbonylation reactions: catalysis by magnetic nanoparticle-supported transition metals.Nanoscale Adv. 2025 Mar 14;7(11):3189-3209. doi: 10.1039/d5na00040h. eCollection 2025 May 27. Nanoscale Adv. 2025. PMID: 40303976 Free PMC article. Review.
-
A Systematic Review of Synthetic and Anticancer and Antimicrobial Activity of Quinazoline/Quinazolin-4-one Analogues.ChemistryOpen. 2025 Jul;14(7):e202400439. doi: 10.1002/open.202400439. Epub 2025 Jan 28. ChemistryOpen. 2025. PMID: 39871708 Free PMC article.
-
Regioselective quinazoline C2 modifications through the azide-tetrazole tautomeric equilibrium.Beilstein J Org Chem. 2024 Mar 28;20:675-683. doi: 10.3762/bjoc.20.61. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 38590535 Free PMC article.
-
Advances in gold catalyzed synthesis of quinoid heteroaryls.RSC Adv. 2024 Jul 3;14(29):21047-21064. doi: 10.1039/d4ra03368j. eCollection 2024 Jun 27. RSC Adv. 2024. PMID: 38962094 Free PMC article. Review.
References
-
- Alsalahi W., Trzeciak A. M. (2021). Rhodium-catalyzed hydroformylation under green conditions: Aqueous/organic biphasic, “on water”, solventless and Rh nanoparticle based systems. Coord. Chem. Rev. 430, 213732–213755. 10.1016/j.ccr.2020.213732 - DOI
-
- Anandaraj P., Ramesh R., Kumaradhas P. (2021). Palladium(ii) N,N,O-pincer type complex-mediated dehydrogenative coupling of alcohols to quinazolines. New J. Chem. 45, 16572–16580. 10.1039/d1nj03146e - DOI
-
- Anwarul Islam A. K. M., Kashem M. A., Shameem I. A., Kibria S. A. M. G. (2005). Efficacy of terazosin and finasteride in symptomatic benign prostatic hyperplasia: A comparative study. Bangladesh Med. Res. Counc. Bull. 31, 54–61. - PubMed
-
- Arachchige P. T. K., Yi C. S. (2019). Synthesis of quinazoline and quinazolinone derivatives via ligand-promoted ruthenium-catalyzed dehydrogenative and deaminative coupling reaction of 2-aminophenyl ketones and 2-aminobenzamides with amines. Org. Lett. 21, 3337–3341. 10.1021/acs.orglett.9b01082 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

