Progression after discontinuation of bevacizumab in the first-line treatment of ovarian cancer
- PMID: 37007537
- PMCID: PMC10061489
- DOI: 10.21037/atm-22-6389
Progression after discontinuation of bevacizumab in the first-line treatment of ovarian cancer
Keywords: BOOST trial; Ovarian cancer; bevacizumab.
Conflict of interest statement
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-6389/coif). NM received a research grant from AstraZeneca. NM also received lecture fees from AstraZeneca, Takeda Pharmaceutical, and Chugai Pharmaceutical. The other author has no conflicts of interest to declare.
Figures
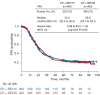
Comment on
-
Optimal Treatment Duration of Bevacizumab as Front-Line Therapy for Advanced Ovarian Cancer: AGO-OVAR 17 BOOST/GINECO OV118/ENGOT Ov-15 Open-Label Randomized Phase III Trial.J Clin Oncol. 2023 Feb 1;41(4):893-902. doi: 10.1200/JCO.22.01010. Epub 2022 Nov 4. J Clin Oncol. 2023. PMID: 36332161 Clinical Trial.
References
Publication types
LinkOut - more resources
Full Text Sources
