Organocatalysis with carbon nitrides
- PMID: 37007670
- PMCID: PMC10054243
- DOI: 10.1080/14686996.2023.2188879
Organocatalysis with carbon nitrides
Abstract
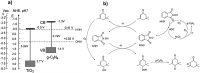
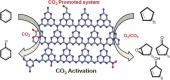
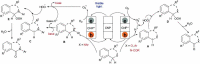
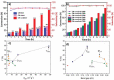
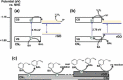
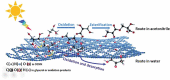
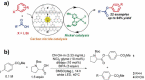
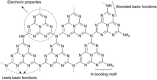
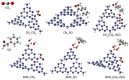
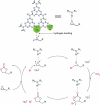
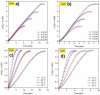
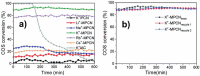
Carbon nitrides, a distinguished class of metal-free catalytic materials, have presented a good potential for chemical transformations and are expected to become prominent materials for organocatalysis. This is largely possible due to their low cost, exceptional thermal and chemical stability, non-toxicity, ease of functionalization, porosity development, etc. Especially, the carbon nitrides with increased porosity and nitrogen contents are more versatile than their bulk counterparts for catalysis. These N-rich carbon nitrides are discussed in the earlier parts of the review. Later, the review highlights the role of such carbon nitride materials for the various organic catalytic reactions including Knoevenagel condensation, oxidation, hydrogenation, esterification, transesterification, cycloaddition, and hydrolysis. The recently emerging concepts in carbon nitride-based organocatalysis have been given special attention. In each of the sections, the structure-property relationship of the materials was discussed and related to their catalysis action. Relevant comparisons with other catalytic materials are also discussed to realize their real potential value. The perspective, challenges, and future directions are also discussed. The overall objective of this review is to provide up-to-date information on new developments in carbon nitride-based organic catalysis reactions that could see them rising as prominent catalytic materials in the future.
Keywords: Carbon nitride; Knoevenagel condensation; cycloaddition; esterification; hydrolysis; organocatalysis.
© 2023 The Author(s). Published by National Institute for Materials Science in partnership with Taylor & Francis Group.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

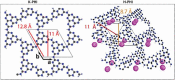
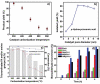
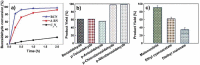
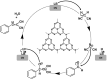




References
-
- Hattori H. Solid base catalysts: fundamentals and their applications in organic reactions. Appl Catal A. 2015;504:103–40.
-
- Gong M, Wang D-Y, Chen C-C, et al. A mini review on nickel-based electrocatalysts for alkaline hydrogen evolution reaction. Nano Res. 2016;9(1):28–46.
-
- Thomas A, Fischer A, Goettmann F, et al. Graphitic carbon nitride materials: variation of structure and morphology and their use as metal-free catalysts. J Mater Chem. 2008;18(41):4893–4908.
-
- Hattori H, Ono Y. Catalysts and catalysis for acid–base reactions. In: Védrine JC, editor. Metal oxides in heterogeneous catalysis. Netherlands: Elsevier; 2018. p. 133–209.
-
- Liu Z, Du Y, Zhang P, et al. Bringing catalytic order out of chaos with nitrogen-doped ordered mesoporous carbon. Matters. 2021;4(10):3161–3194.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
