Serial viral load analysis by DDPCR to evaluate FNC efficacy and safety in the treatment of mild cases of COVID-19
- PMID: 37007788
- PMCID: PMC10053779
- DOI: 10.3389/fmed.2023.1143485
Serial viral load analysis by DDPCR to evaluate FNC efficacy and safety in the treatment of mild cases of COVID-19
Abstract
Introduction: The SARS-CoV-2 outbreak has threatened the human population globally as the numbers of reinfection cases even after large-scale vaccination. Trials have been carried out to find drugs effective in fighting the disease, as COVID-19 is being considered a treatable disease only after we have antivirals. A clinical candidate originally developed for HIV treatment, AZVUDINE (FNC), is a promising drug in the treatment of COVID-19.
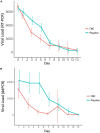
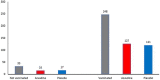
Methods: To predict the clinical outcome of COVID-19, we examined the course of viral load, every 48 h, by RT-PCR, and disease severity using an antiviral drug, FNC, with 281 participants. A randomized clinical trial was performed to evaluate the efficacy of FNC added to standard treatment, compared with placebo group added to standard treatment, for patients with mild COVID-19. RT-qPCR and ddPCR were applied to estimate the viral load in samples from patients. Also, the clinical improvement was evaluated as well as the liver and kidney function.
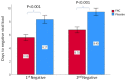
Results and discussion: Notably, the FNC treatment in the mild COVID-19 patients may shorten the time of the nucleic acid negative conversion (NANC) versus placebo group. In addition, the FNC was effective in reducing the viral load of these participants. The present clinical trial results showed that the FNC accelerate the elimination of the virus in and could reduce treatment time of mild patients and save a lot of medical resources, making it a strong candidate for the outpatient and home treatment of COVID-19.
Clinical trial registration: https://clinicaltrials.gov/ct2/show/NCT05033145, identifier NCT05033145.
Keywords: AZVUDINE; COVID-19; FNC; SARS-CoV-2; antiviral.
Copyright © 2023 da Silva, Gebe Abreu Cabral, de Souza, Arruda, Cabral, de Assis, Martins, Tavares, Viana Junior, Chang and Lei.
Conflict of interest statement
This study received funding from HRH Pharmaceutical. The funder had the following involvement with the study: The HRH Pharmaceutical provided all funding for research development. The research was entirely developed by the Galzu Institute (study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication). The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Phase III, randomized, double-blind, placebo-controlled clinical study: a study on the safety and clinical efficacy of AZVUDINE in moderate COVID-19 patients.Front Med (Lausanne). 2023 Oct 19;10:1215916. doi: 10.3389/fmed.2023.1215916. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37928473 Free PMC article.
-
A Randomized, Open-Label, Controlled Clinical Trial of Azvudine Tablets in the Treatment of Mild and Common COVID-19, a Pilot Study.Adv Sci (Weinh). 2020 Oct;7(19):e2001435. doi: 10.1002/advs.202001435. Epub 2020 Aug 13. Adv Sci (Weinh). 2020. PMID: 35403380 Free PMC article.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Advances in the effectiveness and safety of azvudine treatment: a comprehensive review.Front Pharmacol. 2025 Apr 25;16:1524072. doi: 10.3389/fphar.2025.1524072. eCollection 2025. Front Pharmacol. 2025. PMID: 40351412 Free PMC article. Review.
-
Ivermectin for preventing and treating COVID-19.Cochrane Database Syst Rev. 2021 Jul 28;7(7):CD015017. doi: 10.1002/14651858.CD015017.pub2. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 Jun 21;6:CD015017. doi: 10.1002/14651858.CD015017.pub3. PMID: 34318930 Free PMC article. Updated.
Cited by
-
Efficacy and safety evaluation of Azvudine in the prospective treatment of COVID-19 based on four phase III clinical trials.Front Pharmacol. 2023 Aug 24;14:1228548. doi: 10.3389/fphar.2023.1228548. eCollection 2023. Front Pharmacol. 2023. PMID: 37693894 Free PMC article.
-
A Real-World Retrospective Study on the Efficacy and Safety of Four Antiviral Drugs for Hospitalized COVID-19 Patients: Nirmatrelvir/Ritonavir, Simnotrelvir/Ritonavir, Molnupiravir and Azvudine.Infect Drug Resist. 2024 Sep 14;17:3967-3978. doi: 10.2147/IDR.S477083. eCollection 2024. Infect Drug Resist. 2024. PMID: 39296775 Free PMC article.
-
Azvudine efficacy in reducing mortality in COVID-19 patients.Eur J Med Res. 2024 Dec 26;29(1):625. doi: 10.1186/s40001-024-02220-9. Eur J Med Res. 2024. PMID: 39725989 Free PMC article.
-
Effectiveness and Safety of Oral Azvudine for Elderly Hospitalized Patients With COVID-19: A Multicenter, Retrospective, Real-World Study.Adv Sci (Weinh). 2025 Apr;12(13):e2404450. doi: 10.1002/advs.202404450. Epub 2025 Feb 11. Adv Sci (Weinh). 2025. PMID: 39932451 Free PMC article.
-
Inflammatory predictors (eosinophil, C-RP and IL-6) and effectiveness of oral Azvudine tablets treatment in COVID-19 hospitalized patients: A retrospective, self-controlled study.Heliyon. 2023 Nov 7;9(11):e21941. doi: 10.1016/j.heliyon.2023.e21941. eCollection 2023 Nov. Heliyon. 2023. PMID: 38034620 Free PMC article.
References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

