Clinical Characteristics and Pharmacokinetics Change of Long-Term Responders to Antiprogrammed Cell Death Protein 1 Inhibitor Among Patients With Advanced NSCLC
- PMID: 37007867
- PMCID: PMC10050777
- DOI: 10.1016/j.jtocrr.2023.100474
Clinical Characteristics and Pharmacokinetics Change of Long-Term Responders to Antiprogrammed Cell Death Protein 1 Inhibitor Among Patients With Advanced NSCLC
Abstract
Introduction: Immune checkpoint inhibitors (ICIs) induce long-term, durable responses in patients with advanced NSCLC. Nevertheless, these responses are limited to a few patients, and most responders have disease progression. The purpose of this study was to determine the differences in clinical factors and blood drug concentrations between long-term responders (LTRs) and non-LTRs.
Methods: We retrospectively analyzed consecutive patients with advanced NSCLC who received antiprogrammed cell death protein 1 (PD-1) inhibitor monotherapy (nivolumab) from December 22, 2015, to May 31, 2017. Patients who obtained a clinical benefit for more than 6 months were referred to as "responders"; among these, individuals who had a durable response for more than 2 years were defined as "LTRs." Those with a clinical benefit for less than 2 years were defined as "non-LTRs."
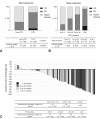
Results: A total of 212 patients received anti-PD-1 inhibitor monotherapy. The responders accounted for 35% (75 of 212) of the patients. Of these, 29 (39%) were LTRs and 46 (61%) were non-LTRs. The overall response rate and median tumor shrinkage in the LTR group were significantly higher than those in the non-LTR group (76% versus 35%, p < 0.0001, and 66% versus 16%, p < 0.001, respectively). The groups had no significant difference in PD-L1 expression and serum drug concentration at 3- and 6-month post-treatment initiation.
Conclusions: Significant tumor shrinkage was associated with a long-term response to an anti-PD-1 inhibitor. Nevertheless, the PD-L1 expression level and pharmacokinetic profile of the inhibitor could not be used to predict the durable response among the responders.
Keywords: Anti–PD-1 inhibitor; Long-term response; Non–small cell lung cancer; Pharmacokinetics.
© 2023 The Authors.
Figures



References
-
- Gandhi L., Rodriguez-Abreu D., Gadgeel S., et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. - PubMed
-
- Herbst R.S., Garon E.B., Kim D.W., et al. Five year survival update from KEYNOTE-010: pembrolizumab versus docetaxel for previously treated, programmed death-ligand 1-positive advanced NSCLC. J Thorac Oncol. 2021;16:1718–1732. - PubMed
-
- Reck M., Rodriguez-Abreu D., Robinson A.G., et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37:537–546. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

