Didanosine-Associated Retinal Toxicity in a Patient With a Mutation in the CRB1 Gene
- PMID: 37007923
- PMCID: PMC9976027
- DOI: 10.1177/24741264211044599
Didanosine-Associated Retinal Toxicity in a Patient With a Mutation in the CRB1 Gene
Abstract
Purpose: This article describes a case of didanosine (DDI)-associated retinal toxicity in a patient with a heterozygous pathogenic variant in the CRB1 gene.
Methods: Case report.
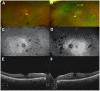
Results: A middle-aged patient with HIV controlled on HAART therapy, and a remote 10-year year history of treatment with DDI and tenofivir, presented with external ophthalmoplegia and well-circumscribed, midperipheral patterns of bilateral pigmentary retinopathy and chorioretinal atrophy in both eyes. Genetic testing revealed a heterozygous pathogenic variant in the CRB1 gene that encodes a protein (Crumbs homolog 1) involved in regulation of cell polarity and junctions and is localized adjacent to mitochondria in the ellipsoid and myoid area.
Conclusions: This case highlights a potential role for genetic susceptibility to retinal toxicity in DDI-associated retinal toxicity. Large, prospective pharmacogenomics studies may be informative to further elucidate the role of genetic risk factors in drug-induced retinal toxicity.
Keywords: CRB1; didanosine; toxicity.
© The Author(s) 2021.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Similar articles
-
DIDANOSINE RETINAL TOXICITY.Retina. 2016 Dec;36 Suppl 1:S159-S167. doi: 10.1097/IAE.0000000000001267. Retina. 2016. PMID: 28005674
-
Envisioning the development of a CRISPR-Cas mediated base editing strategy for a patient with a novel pathogenic CRB1 single nucleotide variant.Ophthalmic Genet. 2022 Oct;43(5):661-670. doi: 10.1080/13816810.2022.2073599. Epub 2022 May 10. Ophthalmic Genet. 2022. PMID: 35538629
-
A Rare Case of Didanosine-Induced Mid-Peripheral Chorioretinal Atrophy Identified Incidentally 11 Years after the Drug Cessation.Medicina (Kaunas). 2022 May 30;58(6):735. doi: 10.3390/medicina58060735. Medicina (Kaunas). 2022. PMID: 35743998 Free PMC article.
-
The Enigma of CRB1 and CRB1 Retinopathies.Adv Exp Med Biol. 2019;1185:251-255. doi: 10.1007/978-3-030-27378-1_41. Adv Exp Med Biol. 2019. PMID: 31884620 Review.
-
CRB1 mutation spectrum in inherited retinal dystrophies.Hum Mutat. 2004 Nov;24(5):355-69. doi: 10.1002/humu.20093. Hum Mutat. 2004. PMID: 15459956 Review.
Cited by
-
Fundus Autofluorescence in Inherited Retinal Disease: A Review.Cells. 2025 Jul 16;14(14):1092. doi: 10.3390/cells14141092. Cells. 2025. PMID: 40710345 Free PMC article. Review.
-
Ocular Manifestations of Perinatal HIV Infection in Kenyan Children on HAART: A Cross-Sectional Comparative Study.Clin Ophthalmol. 2025 Jul 8;19:2143-2151. doi: 10.2147/OPTH.S530332. eCollection 2025. Clin Ophthalmol. 2025. PMID: 40655037 Free PMC article.
References
-
- Gabrielian A, MacCumber MM, Kukuyev A, Mitsuyasu R, Holland GN, Sarraf D. Didanosine-associated retinal toxicity in adults infected with human immunodeficiency virus. JAMA Ophthalmol. 2013;131(2):255–259. doi:10.1001/jamaophthalmol.2013.579 - PubMed
-
- Whitcup SM, Butler KM, Caruso R, et al. Retinal toxicity in human immunodeficiency virus-infected children treated with 2′,3′-dideoxyinosine. Am J Ophthalmol. 1992;113(1):1–7. doi:10.1016/s0002-9394(14)75744-7 - PubMed
-
- Cobo J, Ruiz MF, Figueroa MS, et al. Retinal toxicity associated with didanosine in HIV-infected adults. AIDS. 1996;10(11):1297–1300. doi:10.1097/00002030-199609000-00022 - PubMed
-
- Kearney BP, Sayre JR, Flaherty JF, Chen SS, Kaul S, Cheng AK. Drug-drug and drug-food interactions between tenofovir disoproxil fumarate and didanosine. J Clin Pharmacol. 2005;45(12):1360–1367. doi:10.1177/0091270005281351 - PubMed
Publication types
LinkOut - more resources
Full Text Sources

