Mesenchymal stem cell-derived secretome enhances nucleus pulposus cell metabolism and modulates extracellular matrix gene expression in vitro
- PMID: 37008028
- PMCID: PMC10060656
- DOI: 10.3389/fbioe.2023.1152207
Mesenchymal stem cell-derived secretome enhances nucleus pulposus cell metabolism and modulates extracellular matrix gene expression in vitro
Abstract
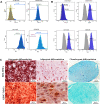
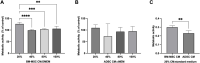
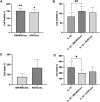
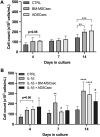
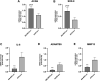
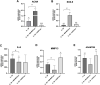
Introduction: Intradiscal mesenchymal stromal cell (MSC) therapies for intervertebral disc degeneration (IDD) have been gaining increasing interest due to their capacity to ameliorate intervertebral disc metabolism and relieve low back pain (LBP). Recently, novel investigations have demonstrated that most of MSC anabolic effects are exerted by secreted growth factors, cytokines, and extracellular vesicles, collectively defined as their secretome. In this study, we aimed to evaluate the effect of bone-marrow-MSCs (BM-MSCs) and adipose-derived stromal cells (ADSCs) secretomes on human nucleus pulposus cells (hNPCs) in vitro. Methods: BM-MSCs and ADSCs were characterized according to surface marker expression by flow cytometry and multilineage differentiation by Alizarin red, Red Oil O and Alcian blue staining. After isolation, hNPCs were treated with either BM-MSC secretome, ADSC secretome, interleukin (IL)-1β followed by BM-MSC secretome or IL-1β followed by ADSC secretome. Cell metabolic activity (MTT assay), cell viability (LIVE/DEAD assay), cell content, glycosaminoglycan production (1,9-dimethylmethylene blue assay), extracellular matrix and catabolic marker gene expression (qPCR) were assessed. Results: 20% BM-MSC and ADSC secretomes (diluted to normal media) showed to exert the highest effect towards cell metabolism and were then used in further experiments. Both BM-MSC and ADSC secretomes improved hNPC viability, increased cell content and enhanced glycosaminoglycan production in basal conditions as well as after IL-1β pretreatment. BM-MSC secretome significantly increased ACAN and SOX9 gene expression, while reducing the levels of IL6, MMP13 and ADAMTS5 both in basal conditions and after in vitro inflammation with IL-1β. Interestingly, under IL-1β stimulation, ADSC secretome showed a catabolic effect with decreased extracellular matrix markers and increased levels of pro-inflammatory mediators. Discussion: Collectively, our results provide new insights on the biological effect of MSC-derived secretomes on hNPCs, with intriguing implications on the development of cell-free approaches to treat IDD.
Keywords: growth factors; intervertebral disc; intervertebral disc degeneration; low back pain; mesenchymal stromal cells; secretome.
Copyright © 2023 Tilotta, Vadalà, Ambrosio, Cicione, Di Giacomo, Russo, Papalia and Denaro.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Cavallo C., Merli G., Zini N., D’Adamo S., Cattini L., Guescini M., et al. (2022). Small extracellular vesicles from inflamed adipose derived stromal cells enhance the NF-κB-Dependent inflammatory/catabolic environment of osteoarthritis. Stem Cells Int. 2022, 1–19. 10.1155/2022/9376338 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

