Potassium Poly(heptazine imide) Coupled with Ti3C2 MXene-Derived TiO2 as a Composite Photocatalyst for Efficient Pollutant Degradation
- PMID: 37008085
- PMCID: PMC10061626
- DOI: 10.1021/acsomega.3c00150
Potassium Poly(heptazine imide) Coupled with Ti3C2 MXene-Derived TiO2 as a Composite Photocatalyst for Efficient Pollutant Degradation
Abstract
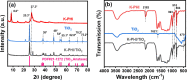
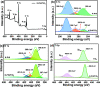
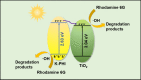
The photocatalytic degradation of pollutants is an effective and sustainable way to solve environmental problems, and the key is to develop an efficient, low-cost, and stable photocatalyst. Polymeric potassium poly(heptazine imide) (K-PHI), as a new member of the carbon nitride family, is a promising candidate but is characterized by a high charge recombination rate. To solve this problem, K-PHI was in-situ composited with MXene Ti3C2-derived TiO2 to construct a type-II heterojunction. The morphology and structure of composite K-PHI/TiO2 photocatalysts were characterized via different technologies, including TEM, XRD, FT-IR, XPS, and UV-vis reflectance spectra. Robust heterostructures and tight interactions between the two components of the composite were verified. Furthermore, the K-PHI/TiO2 photocatalyst showed excellent activity for Rhodamine 6G removal under visible light illumination. When the weight percent of K-PHI in the original mixture of K-PHI and Ti3C2 was set to 10%, the prepared K-PHI/TiO2 composite photocatalyst shows the highest photocatalytic degradation efficiency as high as 96.3%. The electron paramagnetic resonance characterization indicated that the·OH radical is the active species accounting for the degradation of Rhodamine 6G.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Hassani A.; Eghbali P.; Mahdipour F.; Wacławek S.; Lin K.-Y. A.; Ghanbari F. Insights into the synergistic role of photocatalytic activation of peroxymonosulfate by UVA-LED irradiation over CoFe2O4-rGO nanocomposite towards effective Bisphenol A degradation: Performance, mineralization, and activation mechanism. Chem. Eng. J. 2023, 453, 139556 10.1016/j.cej.2022.139556. - DOI
-
- Yaghoot-Nezhad A.; Wacławek S.; Madihi-Bidgoli S.; Hassani A.; Lin K.-Y. A.; Ghanbari F. Heterogeneous photocatalytic activation of electrogenerated chlorine for the production of reactive oxygen and chlorine species: A new approach for Bisphenol A degradation in saline wastewater. J. Hazard. Mater. 2023, 445, 130626 10.1016/j.jhazmat.2022.130626. - DOI - PubMed
-
- Biswal L.; Nayak S.; Parida K. Recent progress on strategies for the preparation of 2D/2D MXene/g-C3N4 nanocomposites for photocatalytic energy and environmental applications. Catal. Sci. Technol. 2021, 11, 1222–1248. 10.1039/D0CY02156C. - DOI
-
- Nayak S.; Parida K. MgCr-LDH nanoplatelets as effective oxidation catalysts for visible light-triggered Rhodamine B degradation. Catalysts 2021, 11, 1072. 10.3390/catal11091072. - DOI
LinkOut - more resources
Full Text Sources

