Transition Metal-Decorated Mg12O12 Nanoclusters as Biosensors and Efficient Drug Carriers for the Metformin Anticancer Drug
- PMID: 37008110
- PMCID: PMC10061506
- DOI: 10.1021/acsomega.3c00058
Transition Metal-Decorated Mg12O12 Nanoclusters as Biosensors and Efficient Drug Carriers for the Metformin Anticancer Drug
Abstract
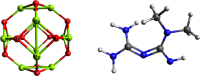
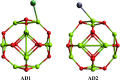
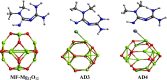
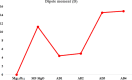
Drug carriers have been designed and investigated remarkably due to their effective use in the modern medication process. In this study, the decoration of the Mg12O12 nanocluster has been done with transition metals (Ni and Zn) for effective adsorption of metformin (anticancer drug). Decoration of Ni and Zn on a nanocluster allows two geometries, and similarly, the adsorption of metformin also provides two geometries. Density functional theory and time-dependent density functional theory have been employed at the B3LYP with 6-311G(d,p) level. The decoration of Ni and Zn offers good attachment and detachment of the drug, which is observed from their good adsorption energy values. Further, the reduction in the energy band gap is noted in the metformin-adsorbed nanocluster, which allows high charge transfer from a lower energy level to a high energy level. The drug carrier systems show an efficient working mechanism in a water solvent with the visible-light absorption range. Natural bonding orbital and dipole moment values suggested that the adsorption of the metformin causes charge separation in these systems. Moreover, low values of chemical softness with a high electrophilic index recommended that these systems are naturally stable with the least reactivity. Thus, we offer novel kinds of Ni- and Zn-decorated Mg12O12 nanoclusters as efficient carriers for metformin and also recommend them to experimentalists for the future development of drug carriers.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
A Theoretical Framework of Zinc-Decorated Inorganic Mg12O12 Nanoclusters for Efficient COCl2 Adsorption: A Step Forward toward the Development of COCl2 Sensing Materials.ACS Omega. 2021 Jul 20;6(30):19435-19444. doi: 10.1021/acsomega.1c01473. eCollection 2021 Aug 3. ACS Omega. 2021. PMID: 34368531 Free PMC article.
-
In Silico Designing of Mg12O12 Nanoclusters with a Late Transition Metal for NO2 Adsorption: An Efficient Approach toward the Development of NO2 Sensing Materials.ACS Omega. 2021 May 21;6(22):14191-14199. doi: 10.1021/acsomega.1c00850. eCollection 2021 Jun 8. ACS Omega. 2021. PMID: 34124442 Free PMC article.
-
Prediction and Understanding: Quantum Chemical Framework of Transition Metals Enclosed in a B12N12 Inorganic Nanocluster for Adsorption and Removal of DDT from the Environment.Inorg Chem. 2021 Jul 19;60(14):10837-10847. doi: 10.1021/acs.inorgchem.1c01760. Epub 2021 Jul 7. Inorg Chem. 2021. PMID: 34231358
-
Designing Novel Zn-Decorated Inorganic B12P12 Nanoclusters with Promising Electronic Properties: A Step Forward toward Efficient CO2 Sensing Materials.ACS Omega. 2020 Jun 19;5(25):15547-15556. doi: 10.1021/acsomega.0c01686. eCollection 2020 Jun 30. ACS Omega. 2020. PMID: 32637830 Free PMC article.
-
Metal Nanoclusters Synthesized in Alkaline Ethylene Glycol: Mechanism and Application.Nanomaterials (Basel). 2023 Jan 30;13(3):565. doi: 10.3390/nano13030565. Nanomaterials (Basel). 2023. PMID: 36770526 Free PMC article. Review.
Cited by
-
Doping Detection Based on the Nanoscale: Biosensing Mechanisms and Applications of Two-Dimensional Materials.Biosensors (Basel). 2025 Apr 3;15(4):227. doi: 10.3390/bios15040227. Biosensors (Basel). 2025. PMID: 40277541 Free PMC article. Review.
-
Theoretical investigation of penicillamine adsorption on mg12o12 fullerene-like cages considering solvent effects, electronic properties, and potential biomedical applications.Sci Rep. 2025 Jul 15;15(1):25547. doi: 10.1038/s41598-025-11275-5. Sci Rep. 2025. PMID: 40665032 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

