Altered neuromagnetic activity in default mode network in childhood absence epilepsy
- PMID: 37008207
- PMCID: PMC10060817
- DOI: 10.3389/fnins.2023.1133064
Altered neuromagnetic activity in default mode network in childhood absence epilepsy
Abstract
Purpose: The electrophysiological characterization of resting state oscillatory functional connectivity within the default mode network (DMN) during interictal periods in childhood absence epilepsy (CAE) remains unclear. Using magnetoencephalographic (MEG) recordings, this study investigated how the connectivity within the DMN was altered in CAE.
Methods: Using a cross-sectional design, we analyzed MEG data from 33 children newly diagnosed with CAE and 26 controls matched for age and sex. The spectral power and functional connectivity of the DMN were estimated using minimum norm estimation combined with the Welch technique and corrected amplitude envelope correlation.
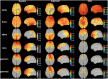
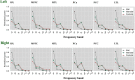
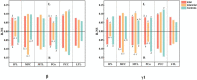
Results: Default mode network showed stronger activation in the delta band during the ictal period, however, the relative spectral power in other bands was significantly lower than that in the interictal period (p corrected < 0.05 for DMN regions, except bilateral medial frontal cortex, left medial temporal lobe, left posterior cingulate cortex in the theta band, and the bilateral precuneus in the alpha band). It should be noted that the significant power peak in the alpha band was lost compared with the interictal data. Compared with controls, the interictal relative spectral power of DMN regions (except bilateral precuneus) in CAE patients was significantly increased in the delta band (p corrected < 0.01), whereas the values of all DMN regions in the beta-gamma 2 band were significantly decreased (p corrected < 0.01). In the higher frequency band (alpha-gamma1), especially in the beta and gamma1 band, the ictal node strength of DMN regions except the left precuneus was significantly higher than that in the interictal periods (p corrected < 0.01), and the node strength of the right inferior parietal lobe increased most significantly in the beta band (Ictal: 3.8712 vs. Interictal: 0.7503, p corrected < 0.01). Compared with the controls, the interictal node strength of DMN increased in all frequency bands, especially the right medial frontal cortex in the beta band (Controls: 0.1510 vs. Interictal: 3.527, p corrected < 0.01). Comparing relative node strength between groups, the right precuneus in CAE children decreased significantly (β: Controls: 0.1009 vs. Interictal: 0.0475; γ 1: Controls:0.1149 vs. Interictal:0.0587, p corrected < 0.01) such that it was no longer the central hub.
Conclusion: These findings indicated DMN abnormalities in CAE patients, even in interictal periods without interictal epileptic discharges. Abnormal functional connectivity in CAE may reflect abnormal anatomo-functional architectural integration in DMN, as a result of cognitive mental impairment and unconsciousness during absence seizure. Future studies are needed to examine if the altered functional connectivity can be used as a biomarker for treatment responses, cognitive dysfunction, and prognosis in CAE patients.
Keywords: childhood absence epilepsy; functional connectivity; ictal; interictal; magnetoencephalography; oscillations; spectral power.
Copyright © 2023 Wang, Li, Sun, Xu, Xu, Wang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Alterations in the default mode network in rolandic epilepsy with mild spike-wave index in non-rapid eye movement sleep.Front Neurosci. 2022 Aug 9;16:944391. doi: 10.3389/fnins.2022.944391. eCollection 2022. Front Neurosci. 2022. PMID: 36017188 Free PMC article.
-
Increased Intrinsic Connectivity of the Default Mode Network in Temporal Lobe Epilepsy: Evidence from Resting-State MEG Recordings.PLoS One. 2015 Jun 2;10(6):e0128787. doi: 10.1371/journal.pone.0128787. eCollection 2015. PLoS One. 2015. PMID: 26035750 Free PMC article.
-
Differences in generation and maintenance between ictal and interictal generalized spike-and-wave discharges in childhood absence epilepsy: A magnetoencephalography study.Epilepsy Behav. 2023 Nov;148:109440. doi: 10.1016/j.yebeh.2023.109440. Epub 2023 Sep 23. Epilepsy Behav. 2023. PMID: 37748416
-
Presurgical Evaluation of Epilepsy Using Resting-State MEG Functional Connectivity.Front Hum Neurosci. 2021 Jul 2;15:649074. doi: 10.3389/fnhum.2021.649074. eCollection 2021. Front Hum Neurosci. 2021. PMID: 34276321 Free PMC article. Review.
-
The Oscillatory Basis of Working Memory Function and Dysfunction in Epilepsy.Front Hum Neurosci. 2021 Jan 12;14:612024. doi: 10.3389/fnhum.2020.612024. eCollection 2020. Front Hum Neurosci. 2021. PMID: 33584224 Free PMC article. Review.
Cited by
-
Understanding of Consciousness in Absence Seizures: A Literature Review.Neuropsychiatr Dis Treat. 2024 Jun 24;20:1345-1353. doi: 10.2147/NDT.S391052. eCollection 2024. Neuropsychiatr Dis Treat. 2024. PMID: 38947367 Free PMC article. Review.
-
Frontal lobe epilepsy: an eye tracking study of memory and attention.Front Neurosci. 2023 Dec 5;17:1298468. doi: 10.3389/fnins.2023.1298468. eCollection 2023. Front Neurosci. 2023. PMID: 38116071 Free PMC article.
-
Involvement of the default mode network in patients with transient global amnesia: multilayer network.Neuroradiology. 2023 Dec;65(12):1729-1736. doi: 10.1007/s00234-023-03241-7. Epub 2023 Oct 17. Neuroradiology. 2023. PMID: 37848740
-
Olfactory Epithelium Stimulation Using Rhythmic Nasal Air-Puffs Improves the Cognitive Performance of Individuals with Acute Sleep Deprivation.Brain Sci. 2024 Apr 13;14(4):378. doi: 10.3390/brainsci14040378. Brain Sci. 2024. PMID: 38672027 Free PMC article.
References
-
- Almubarak S., Alexopoulos A., Von-Podewils F., Wang Z. I., Kakisaka Y., Mosher J. C., et al. (2014). The correlation of magnetoencephalography to intracranial EEG in localizing the epileptogenic zone: A study of the surgical resection outcome. Epilepsy Res. 108 1581–1590. 10.1016/j.eplepsyres.2014.08.016 - DOI - PubMed
LinkOut - more resources
Full Text Sources

