Exogenous abscisic acid and sodium nitroprusside regulate flavonoid biosynthesis and photosynthesis of Nitraria tangutorum Bobr in alkali stress
- PMID: 37008502
- PMCID: PMC10057120
- DOI: 10.3389/fpls.2023.1118984
Exogenous abscisic acid and sodium nitroprusside regulate flavonoid biosynthesis and photosynthesis of Nitraria tangutorum Bobr in alkali stress
Abstract
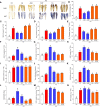
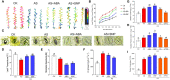
Abscisic acid (ABA) and nitric oxide (NO) are involved in mediating abiotic stress-induced plant physiological responses. Nitraria tangutorum Bobr is a typical salinized desert plant growing in an arid environment. In this study, we investigated the effects of ABA and NO on N.tangutorum seedlings under alkaline stress. Alkali stress treatment caused cell membrane damage, increased electrolyte leakage, and induced higher production of reactive oxygen species (ROS), which caused growth inhibition and oxidative stress in N.tangutorum seedlings. Exogenous application of ABA (15μm) and Sodium nitroprusside (50μm) significantly increased the plant height, fresh weight, relative water content, and degree of succulency in N.tangutorum seedlings under alkali stress. Meanwhile, the contents of ABA and NO in plant leaves were significantly increased. ABA and SNP can promote stomatal closure, decrease the water loss rate, increase leaf surface temperature and the contents of osmotic regulator proline, soluble protein, and betaine under alkali stress. Meanwhile, SNP more significantly promoted the accumulation of chlorophyll a/b and carotenoids, increased quantum yield of photosystem II (φPSII) and electron transport rate (ETRII) than ABA, and decreased photochemical quenching (qP), which improved photosynthetic efficiency and accelerated the accumulation of soluble sugar, glucose, fructose, sucrose, starch, and total sugar. However, compared with exogenous application of SNP in the alkaline stress, ABA significantly promoted the transcription of NtFLS/NtF3H/NtF3H/NtANR genes and the accumulation of naringin, quercetin, isorhamnetin, kaempferol, and catechin in the synthesis pathway of flavonoid metabolites, and isorhamnetin content was the highest. These results indicate that both ABA and SNP can reduce the growth inhibition and physiological damage caused by alkali stress. Among them, SNP has a better effect on the improvement of photosynthetic efficiency and the regulation of carbohydrate accumulation than ABA, while ABA has a more significant effect on the regulation of flavonoid and anthocyanin secondary metabolite accumulation. Exogenous application of ABA and SNP also improved the antioxidant capacity and the ability to maintain Na+/K+ balance of N. tangutorum seedlings under alkali stress. These results demonstrate the beneficial effects of ABA and NO as stress hormones and signaling molecules that positively regulate the defensive response of N. tangutorum to alkaline stress.
Keywords: ABA and NO; Nitraria tangutorum Bobr; alkali stress; anthocyanin accumulation; flavone pathway; photosynthetic efficiency.
Copyright © 2023 Zhang, Cheng, Liu, Dai, Zheng and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
LinkOut - more resources
Full Text Sources

