Silencing circPalm2 inhibits sepsis-induced acute lung injury by sponging miR-376b-3p and targeting MAP3K1
- PMID: 37008689
- PMCID: PMC10050541
- DOI: 10.1007/s43188-022-00169-7
Silencing circPalm2 inhibits sepsis-induced acute lung injury by sponging miR-376b-3p and targeting MAP3K1
Abstract
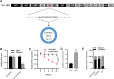
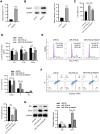
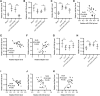
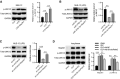
The apoptosis and inflammation of pulmonary epithelial cells are important pathogenic factors of sepsis-induced acute lung injury (ALI). Upregulation of circPalm2 (circ_0001212) expression levels has been previously detected in the lung tissue of ALI rats. Herein, the biological significance and detailed mechanism of circPalm2 in ALI pathogenesis were investigated. In vivo models of sepsis-induced ALI were established by treating C57BL/6 mice with cecal ligation and puncture (CLP) surgery. Murine pulmonary epithelial cells (MLE-12 cells) were stimulated with lipopolysaccharide (LPS) to establish in vitro septic ALI models. MLE-12 cell viability and apoptosis were evaluated by CCK-8 assay and flow cytometry analysis, respectively. The pathological alterations of the lung tissue were analysed based on hematoxylin-eosin (H&E) staining. Cell apoptosis in the lung tissue samples was examined by TUNEL staining assay. LPS administration suppressed the viability and accelerated the inflammation and apoptotic behaviours of MLE-12 cells. CircPalm2 displayed high expression in LPS-stimulated MLE-12 cells and possessed circular characteristics. The silencing of circPalm2 impeded apoptosis and inflammation in LPS-stimulated MLE-12 cells. Mechanistically, circPalm2 bound with miR-376b-3p, which targeted MAP3K1. In rescue assays, MAP3K1 enhancement reversed the repressive effects of circPalm2 depletion on LPS-triggered inflammatory injury and MLE-12 cell apoptosis. Furthermore, the lung tissue collected from CLP model mice displayed low miR-376b-3p expression and high levels of circPalm2 and MAP3K1. CircPalm2 positively regulated MAP3K1 expression by downregulating miR-376b-3p in murine lung tissues. Importantly, circPalm2 knockdown attenuated CLP-induced inflammation, apoptosis, and pathological alterations in lung tissues collected from mice. Silenced circPalm2 inhibits LPS-induced pulmonary epithelial cell dysfunction and mitigates abnormalities in lung tissues collected from CLP-stimulated mice via the miR-376b-3p/MAP3K1 axis in septic ALI.
Supplementary information: The online version contains supplementary material available at 10.1007/s43188-022-00169-7.
Keywords: Acute lung injury; Inflammation; MAP3K1; Sepsis; circPalm2; miR-376b-3p.
© The Author(s) under exclusive licence to Korean Society of Toxicology 2023, Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Conflict of interestThe authors declare no conflicts of interest.
Figures







Similar articles
-
Knockdown of circRNA Paralemmin 2 Ameliorates Lipopolysaccharide-induced Murine Lung Epithelial Cell Injury by Sponging miR-330-5p to Reduce ROCK2 Expression.Immunol Invest. 2022 Aug;51(6):1707-1724. doi: 10.1080/08820139.2022.2027961. Epub 2022 Feb 16. Immunol Invest. 2022. PMID: 35171050
-
CircPalm2 knockdown alleviates LPS-evoked pulmonary microvascular endothelial cell apoptosis and inflammation via miR-450b-5p/ROCK1 axis.Int Immunopharmacol. 2022 Dec;113(Pt A):109199. doi: 10.1016/j.intimp.2022.109199. Epub 2022 Oct 14. Int Immunopharmacol. 2022. PMID: 36252478
-
circRNA_0001679/miR-338-3p/DUSP16 axis aggravates acute lung injury.Open Med (Wars). 2022 Feb 28;17(1):403-413. doi: 10.1515/med-2022-0417. eCollection 2022. Open Med (Wars). 2022. Retraction in: Open Med (Wars). 2023 Dec 31;18(1):20230891. doi: 10.1515/med-2023-0891. PMID: 35291714 Free PMC article. Retracted.
-
Calycosin Attenuates Lipopolysaccharide-Induced Acute Lung Injury in Mice through the miR-375-3p/ROCK2 Axis.J Invest Surg. 2023 Dec;36(1):2211166. doi: 10.1080/08941939.2023.2211166. J Invest Surg. 2023. PMID: 37400250
-
Role and mechanisms of autophagy, ferroptosis, and pyroptosis in sepsis-induced acute lung injury.Front Pharmacol. 2024 Aug 5;15:1415145. doi: 10.3389/fphar.2024.1415145. eCollection 2024. Front Pharmacol. 2024. PMID: 39161900 Free PMC article. Review.
Cited by
-
Serotype-Dependent Inhibition of Streptococcus pneumoniae Growth by Short-Chain Fatty Acids.J Microbiol Biotechnol. 2024 Jan 28;34(1):47-55. doi: 10.4014/jmb.2309.09003. Epub 2023 Nov 20. J Microbiol Biotechnol. 2024. PMID: 38044707 Free PMC article.
-
Bacillus subtilis Induces Human Beta Defensin-2 Through its Lipoproteins in Human Intestinal Epithelial Cells.Probiotics Antimicrob Proteins. 2025 Jun;17(3):1648-1662. doi: 10.1007/s12602-024-10224-4. Epub 2024 Feb 20. Probiotics Antimicrob Proteins. 2025. PMID: 38376819 Free PMC article.
-
HMGB1 inhibition blocks ferroptosis and oxidative stress to ameliorate sepsis-induced acute lung injury by activating the Nrf2 pathway.Kaohsiung J Med Sci. 2024 Aug;40(8):710-721. doi: 10.1002/kjm2.12851. Epub 2024 Jun 5. Kaohsiung J Med Sci. 2024. PMID: 38837857 Free PMC article.
-
Tagetes erecta Linn flower extract inhibits particulate matter 2.5-promoted epithelial-mesenchymal transition by attenuating reactive oxygen species generation in human retinal pigment epithelial ARPE-19 cells.Nutr Res Pract. 2025 Apr;19(2):170-185. doi: 10.4162/nrp.2025.19.2.170. Epub 2024 Dec 4. Nutr Res Pract. 2025. PMID: 40226757 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

