Exogenous L-lactate administration in rat hippocampus increases expression of key regulators of mitochondrial biogenesis and antioxidant defense
- PMID: 37008779
- PMCID: PMC10062455
- DOI: 10.3389/fnmol.2023.1117146
Exogenous L-lactate administration in rat hippocampus increases expression of key regulators of mitochondrial biogenesis and antioxidant defense
Abstract
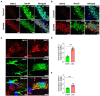
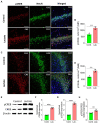
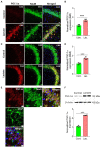
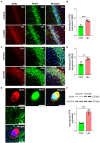
L-lactate plays a critical role in learning and memory. Studies in rats showed that administration of exogenous L-lactate into the anterior cingulate cortex and hippocampus (HPC) improved decision-making and enhanced long-term memory formation, respectively. Although the molecular mechanisms by which L-lactate confers its beneficial effect are an active area of investigations, one recent study found that L-lactate supplementation results in a mild reactive oxygen species burst and induction of pro-survival pathways. To further investigate the molecular changes induced by L-lactate, we injected rats with either L-lactate or artificial CSF bilaterally into the dorsal HPC and collected the HPC after 60 minutes for mass spectrometry. We identified increased levels of several proteins that include SIRT3, KIF5B, OXR1, PYGM, and ATG7 in the HPC of the L-lactate treated rats. SIRT3 (Sirtuin 3) is a key regulator of mitochondrial functions and homeostasis and protects cells against oxidative stress. Further experiments identified increased expression of the key regulator of mitochondrial biogenesis (PGC-1α) and mitochondrial proteins (ATPB, Cyt-c) as well as increased mitochondrial DNA (mtDNA) copy number in the HPC of L-lactate treated rats. OXR1 (Oxidation resistance protein 1) is known to maintain mitochondrial stability. It mitigates the deleterious effects of oxidative damage in neurons by inducing a resistance response against oxidative stress. Together, our study suggests that L-lactate can induce expression of key regulators of mitochondrial biogenesis and antioxidant defense. These findings create new research avenues to explore their contribution to the L-lactate's beneficial effect in cognitive functions as these cellular responses might enable neurons to generate more ATP to meet energy demand of neuronal activity and synaptic plasticity as well as attenuate the associated oxidative stress.
Keywords: PGC-1 alpha; SIRT3; hippocampus; lactate; mitochondrial biogenesis; oxidative stress; proteomics.
Copyright © 2023 Akter, Ma, Hasan, Karim, Zhu, Zhang and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Baeken M. W., Schwarz M., Kern A., Moosmann B., Hajieva P., Behl C. (2021). The selective degradation of sirtuins via macroautophagy in the MPP(+) model of Parkinson's disease is promoted by conserved oxidation sites. Cell Death Discov. 7:286. doi: 10.1038/s41420-021-00683-x, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

