Complex Coacervate Materials as Artificial Cells
- PMID: 37009061
- PMCID: PMC10043873
- DOI: 10.1021/accountsmr.2c00239
Complex Coacervate Materials as Artificial Cells
Abstract
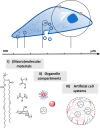
Cells have evolved to be self-sustaining compartmentalized systems that consist of many thousands of biomolecules and metabolites interacting in complex cycles and reaction networks. Numerous subtle intricacies of these self-assembled structures are still largely unknown. The importance of liquid-liquid phase separation (both membraneless and membrane bound) is, however, recognized as playing an important role in achieving biological function that is controlled in time and space. Reconstituting biochemical reactions in vitro has been a success of the last decades, for example, establishment of the minimal set of enzymes and nutrients able to replicate cellular activities like the in vitro transcription translation of genes to proteins. Further than this though, artificial cell research has the aim of combining synthetic materials and nonliving macromolecules into ordered assemblies with the ability to carry out more complex and ambitious cell-like functions. These activities can provide insights into fundamental cell processes in simplified and idealized systems but could also have an applied impact in synthetic biology and biotechnology in the future. To date, strategies for the bottom-up fabrication of micrometer scale life-like artificial cells have included stabilized water-in-oil droplets, giant unilamellar vesicles (GUV's), hydrogels, and complex coacervates. Water-in-oil droplets are a valuable and easy to produce model system for studying cell-like processes; however, the lack of a crowded interior can limit these artificial cells in mimicking life more closely. Similarly membrane stabilized vesicles, such as GUV's, have the additional membrane feature of cells but still lack a macromolecularly crowded cytoplasm. Hydrogel-based artificial cells have a macromolecularly dense interior (although cross-linked) that better mimics cells, in addition to mechanical properties more similar to the viscoelasticity seen in cells but could be seen as being not dynamic in nature and limiting to the diffusion of biomolecules. On the other hand, liquid-liquid phase separated complex coacervates are an ideal platform for artificial cells as they can most accurately mimic the crowded, viscous, highly charged nature of the eukaryotic cytoplasm. Other important key features that researchers in the field target include stabilizing semipermeable membranes, compartmentalization, information transfer/communication, motility, and metabolism/growth. In this Account, we will briefly cover aspects of coacervation theory and then outline key cases of synthetic coacervate materials used as artificial cells (ranging from polypeptides, modified polysaccharides, polyacrylates, and polymethacrylates, and allyl polymers), finishing with envisioned opportunities and potential applications for coacervate artificial cells moving forward.
© 2023 The Authors. Co-published by ShanghaiTech University and American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- van Stevendaal M. H. M. E.; van Hest J. C. M.; Mason A. F. Functional Interactions Between Bottom-Up Synthetic Cells and Living Matter for Biomedical Applications. ChemSystemsChem. 2021, 3 (5), e2100009. 10.1002/syst.202100009. - DOI
-
- Insua I.; Montenegro J. Synthetic Supramolecular Systems in Life-like Materials and Protocell Models. Chem. 2020, 6 (7), 1652–1682. 10.1016/j.chempr.2020.06.005. - DOI
LinkOut - more resources
Full Text Sources
