A randomized controlled trial of varenicline and brief behavioral counseling delivered by lay counselors for adolescent vaping cessation: Study protocol
- PMID: 37009114
- PMCID: PMC10050714
- DOI: 10.3389/fpsyt.2023.1083791
A randomized controlled trial of varenicline and brief behavioral counseling delivered by lay counselors for adolescent vaping cessation: Study protocol
Abstract
Background: Approximately one-fifth of high-school seniors and college students currently vape nicotine. Adolescents express a desire to quit vaping, and case reports have shown promise for e-cigarette tapering with dual behavioral and pharmacologic therapies. However, there are no published clinical trials to date that test these intervention approaches for adolescent nicotine vaping cessation. In this three-arm randomized, placebo-controlled, parallel-group study, we aim to assess the efficacy of varenicline in combination with brief behavioral counseling and texting support on vaping cessation in adolescents dependent on vaped nicotine.
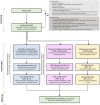
Methods: The study will enroll 300 individuals between the ages of 16-25 with daily or near-daily nicotine vaping who reside in the Greater Boston area. Participants will be randomly assigned in a 1:1:1 ratio in blocks of six to one of the three arms: (1) a 12-week course of varenicline titrated to 1 mg bid, brief behavioral counseling delivered by a lay counselor, and an introduction to This is Quitting (TIQ) texting support created by the Truth Initiative; (2) a 12-week course of placebo, brief behavioral counseling, and TIQ; and (3) 12 weeks of enhanced usual care, consisting of advice to quit and an introduction to TIQ. The primary outcome will be biochemically verified continuous vaping abstinence at the end of the treatment (week 12). Secondary outcomes include continuous abstinence at follow-up (week 24), 7-day point prevalence abstinence at weeks 12 and 24, safety and tolerability of varenicline in an adolescent vaping population, as well as change in mood and nicotine withdrawal symptoms across the intervention period. Exploratory outcomes include change in comorbid substance use behaviors and nicotine dependence. Analysis will be intent-to-treat, with multiple imputation sensitivity analyses for participants with missing or incomplete outcome data.
Discussion: This is the first study to evaluate varenicline in combination with a novel, brief, lay counselor delivered vaping cessation program for adolescents who vape nicotine. Results will inform clinicians on the effectiveness and acceptability of this promising, but not yet tested intervention.Clinical trial registration: ClinicalTrials.gov, identifier NCT05367492.
Keywords: adolescents; cessation; clinical trial; e-cigarette; nicotine; vaping; varenicline; young adults.
Copyright © 2023 Schuster, Cather, Pachas, Nielsen, Iroegbulem, Dufour, Potterx, Levy, Gray and Evins.
Conflict of interest statement
SL serves as an expert witness in the case against JUUL. KG has provided consultation to Pfizer, Inc., and Jazz Pharmaceuticals. AE receives NIDA Grant subcontracts from Brain Solutions, and Charles River Analytics, is on the Data Safety and Monitoring Board for Karuna Pharmaceuticals, and performs Advisory Board work for Alkermes. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Varenicline for Youth Nicotine Vaping Cessation: A Randomized Clinical Trial.JAMA. 2025 Jun 3;333(21):1876-1886. doi: 10.1001/jama.2025.3810. JAMA. 2025. PMID: 40266580 Clinical Trial.
-
Varenicline and counseling for vaping cessation: a double-blind, randomized, parallel-group, placebo-controlled trial.BMC Med. 2023 Jul 5;21(1):220. doi: 10.1186/s12916-023-02919-2. BMC Med. 2023. PMID: 37403047 Free PMC article. Clinical Trial.
-
Effect of Combination Treatment With Varenicline and Nicotine Patch on Smoking Cessation Among Smokers Who Drink Heavily: A Randomized Clinical Trial.JAMA Netw Open. 2022 Mar 1;5(3):e220951. doi: 10.1001/jamanetworkopen.2022.0951. JAMA Netw Open. 2022. PMID: 35244704 Free PMC article. Clinical Trial.
-
Interventions for waterpipe smoking cessation.Cochrane Database Syst Rev. 2023 Jun 7;6(6):CD005549. doi: 10.1002/14651858.CD005549.pub4. Cochrane Database Syst Rev. 2023. PMID: 37286509 Free PMC article. Review.
-
Varenicline: a first-line treatment option for smoking cessation.Clin Ther. 2009 Mar;31(3):463-91. doi: 10.1016/j.clinthera.2009.03.021. Clin Ther. 2009. PMID: 19393839 Review.
Cited by
-
Varenicline for Youth Nicotine Vaping Cessation: A Randomized Clinical Trial.JAMA. 2025 Jun 3;333(21):1876-1886. doi: 10.1001/jama.2025.3810. JAMA. 2025. PMID: 40266580 Clinical Trial.
References
-
- Schulenberg JE, Patrick ME, Johnston LD, O’Malley PM, Bachman JG, Miech RA. Monitoring the Future National Survey Results on Drug Use, 1975-2020, II, College Students & Adults Ages 19-60. Inst Soc Res. (2021) 1:99–147.
-
- Miech RA, Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE, Patrick ME. Monitoring the future National Survey Results on drug use, 1975–2021: Volume 1, Secondary School Students. Inst Soc Res. (2022) 2:30–57.
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

