Retinopathy of prematurity and neurodevelopmental outcomes in preterm infants: A systematic review and meta-analysis
- PMID: 37009271
- PMCID: PMC10050340
- DOI: 10.3389/fped.2023.1055813
Retinopathy of prematurity and neurodevelopmental outcomes in preterm infants: A systematic review and meta-analysis
Abstract
Background: Retinopathy of prematurity (ROP) and abnormal brain development share similar risk factors and mechanisms. There has been contrasting evidence on the association of ROP with adverse neurodevelopmental outcomes.
Objective: We analysed the association between ROP at levels of severity and treatment with all neurodevelopmental outcomes until adolescence.
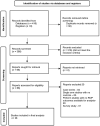
Data source: We followed PRISMA guidelines and searched Medline and Embase between 1 August 1990 and 31 March 2022.
Study selection and participants: Randomised or quasi-randomised clinical trials and observational studies on preterm infants (<37 weeks) with ROP [type 1 or severe ROP, type 2 or milder ROP, laser or anti-vascular endothelial growth factor (VEGF) treated] were included.
Data extraction and synthesis: We included studies on ROP and any neurocognitive or neuropsychiatric outcomes.
Outcomes: The primary outcomes were as follows: cognitive composite scores evaluated between the ages of 18 and 48 months by the Bayley Scales of Infant and Toddler Development (BSID) or equivalent; neurodevelopmental impairment (NDI; moderate to severe NDI or severe NDI), cerebral palsy, cognitive impairment; and neuropsychiatric or behavioural problems. The secondary outcomes were as follows: motor and language composite scores evaluated between the ages of 18 and 48 months by BSID or equivalent; motor/language impairment; and moderate/severe NDI as defined by the authors.
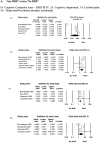
Results: In preterm infants, "any ROP" was associated with an increased risk of cognitive impairment or intellectual disability [n = 83,506; odds ratio (OR): 2.56; 95% CI: 1.40-4.69; p = 0.002], cerebral palsy (n = 3,706; OR: 2.26; 95% CI: 1.72-2.96; p < 0.001), behavioural problems (n = 81,439; OR: 2.45; 95% CI: 1.03-5.83; p = 0.04), or NDI as defined by authors (n = 1,930; OR: 3.83; 95% CI: 1.61-9.12; p = 0.002). Type 1 or severe ROP increased the risk of cerebral palsy (OR: 2.19; 95% CI: 1.23-3.88; p = 0.07), cognitive impairment or intellectual disability (n = 5,167; OR: 3.56; 95% CI: 2.6-4.86; p < 0.001), and behavioural problems (n = 5,500; OR: 2.76; 95% CI: 2.11-3.60; p < 0.001) more than type 2 ROP at 18-24 months. Infants treated with anti-VEGF had higher odds of moderate cognitive impairment than the laser surgery group if adjusted data (gestational age, sex severe intraventricular haemorrhage, bronchopulmonary dysplasia, sepsis, surgical necrotising enterocolitis, and maternal education) were analysed [adjusted OR (aOR): 1.93; 95% CI: 1.23-3.03; p = 0.04], but not for cerebral palsy (aOR: 1.29; 95% CI: 0.65-2.56; p = 0.45). All outcomes were adjudged with a "very low" certainty of evidence.
Conclusion and relevance: Infants with "any ROP" had higher risks of cognitive impairment or intellectual disability, cerebral palsy, and behavioural problems. Anti-VEGF treatment increased the risk of moderate cognitive impairment. These results support the association of ROP and anti-VEGF treatment with adverse neurodevelopmental outcomes.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier: CRD42022326009.
Keywords: anti-VEGF; behavioural issues; bevacizumab; cerebral palsy; preterm; ranibizumab; retinopathy of prematurity.
© 2023 Diggikar, Gurumoorthy, Trif, Mudura, Nagesh, Galis, Vinekar and Kramer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources

