Salmonella enhances osteogenic differentiation in adipose-derived mesenchymal stem cells
- PMID: 37009487
- PMCID: PMC10055666
- DOI: 10.3389/fcell.2023.1077350
Salmonella enhances osteogenic differentiation in adipose-derived mesenchymal stem cells
Abstract
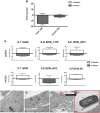
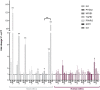
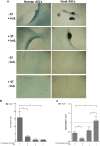
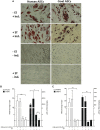
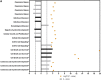
The potential of mesenchymal stem cells (MSCs) for tissue repair and regeneration has garnered great attention. While MSCs are likely to interact with microbes at sites of tissue damage and inflammation, like in the gastrointestinal system, the consequences of pathogenic association on MSC activities have yet to be elucidated. This study investigated the effects of pathogenic interaction on MSC trilineage differentiation paths and mechanisms using model intracellular pathogen Salmonella enterica ssp enterica serotype Typhimurium. The examination of key markers of differentiation, apoptosis, and immunomodulation demonstrated that Salmonella altered osteogenic and chondrogenic differentiation pathways in human and goat adipose-derived MSCs. Anti-apoptotic and pro-proliferative responses were also significantly upregulated (p < 0.05) in MSCs during Salmonella challenge. These results together indicate that Salmonella, and potentially other pathogenic bacteria, can induce pathways that influence both apoptotic response and functional differentiation trajectories in MSCs, highlighting that microbes have a potentially significant role as influencers of MSC physiology and immune activity.
Keywords: MSC; apoptosis; cell death; host/bacteria interactions; pathogenic and infectious disease.
Copyright © 2023 Mohamad-Fauzi, Shaw, Foutouhi, Hess, Kong, Kol, Storey, Desai, Shah, Borjesson, Murray and Weimer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Gain of CXCR7 function with mesenchymal stem cell therapy ameliorates experimental arthritis via enhancing tissue regeneration and immunomodulation.Stem Cell Res Ther. 2021 May 29;12(1):314. doi: 10.1186/s13287-021-02402-w. Stem Cell Res Ther. 2021. PMID: 34051857 Free PMC article.
-
Gastrointestinal microbes interact with canine adipose-derived mesenchymal stem cells in vitro and enhance immunomodulatory functions.Stem Cells Dev. 2014 Aug 15;23(16):1831-43. doi: 10.1089/scd.2014.0128. Epub 2014 Jun 26. Stem Cells Dev. 2014. PMID: 24803072 Free PMC article.
-
Tissue source determines the differentiation potentials of mesenchymal stem cells: a comparative study of human mesenchymal stem cells from bone marrow and adipose tissue.Stem Cell Res Ther. 2017 Dec 6;8(1):275. doi: 10.1186/s13287-017-0716-x. Stem Cell Res Ther. 2017. PMID: 29208029 Free PMC article.
-
Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome.Cells. 2019 May 16;8(5):467. doi: 10.3390/cells8050467. Cells. 2019. PMID: 31100966 Free PMC article. Review.
-
Potential of Using Infrapatellar-Fat-Pad-Derived Mesenchymal Stem Cells for Therapy in Degenerative Arthritis: Chondrogenesis, Exosomes, and Transcription Regulation.Biomolecules. 2022 Mar 1;12(3):386. doi: 10.3390/biom12030386. Biomolecules. 2022. PMID: 35327578 Free PMC article. Review.
Cited by
-
Impact of Different Cell Types on the Osteogenic Differentiation Process of Mesenchymal Stem Cells.Stem Cells Int. 2025 Feb 13;2025:5551222. doi: 10.1155/sci/5551222. eCollection 2025. Stem Cells Int. 2025. PMID: 39980864 Free PMC article. Review.
-
The role of toll-like receptors in orchestrating osteogenic differentiation of mesenchymal stromal cells and osteoimmunology.Front Cell Dev Biol. 2023 Oct 23;11:1277686. doi: 10.3389/fcell.2023.1277686. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37941898 Free PMC article. Review.

