Proteomic analysis defines the interactome of telomerase in the protozoan parasite, Trypanosoma brucei
- PMID: 37009488
- PMCID: PMC10061497
- DOI: 10.3389/fcell.2023.1110423
Proteomic analysis defines the interactome of telomerase in the protozoan parasite, Trypanosoma brucei
Abstract
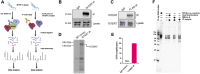
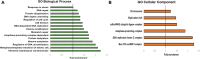
Telomerase is a ribonucleoprotein enzyme responsible for maintaining the telomeric end of the chromosome. The telomerase enzyme requires two main components to function: the telomerase reverse transcriptase (TERT) and the telomerase RNA (TR), which provides the template for telomeric DNA synthesis. TR is a long non-coding RNA, which forms the basis of a large structural scaffold upon which many accessory proteins can bind and form the complete telomerase holoenzyme. These accessory protein interactions are required for telomerase activity and regulation inside cells. The interacting partners of TERT have been well studied in yeast, human, and Tetrahymena models, but not in parasitic protozoa, including clinically relevant human parasites. Here, using the protozoan parasite, Trypanosoma brucei (T. brucei) as a model, we have identified the interactome of T. brucei TERT (TbTERT) using a mass spectrometry-based approach. We identified previously known and unknown interacting factors of TbTERT, highlighting unique features of T. brucei telomerase biology. These unique interactions with TbTERT, suggest mechanistic differences in telomere maintenance between T. brucei and other eukaryotes.
Keywords: Trypanosoma brucei; cell growth; cell proliferation; interactome; parasite; ribonucleoprotein (RNP); telomerase; telomere.
Copyright © 2023 Davis, Reyes, Nitika, Saha, Wolfgeher, Xu, Truman, Li and Chakrabarti.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Ale-Agha N., Jakobs P., Goy C., Zurek M., Rosen J., Dyballa-Rukes N., et al. (2021). Mitochondrial telomerase reverse transcriptase protects from myocardial ischemia/reperfusion injury by improving complex I composition and function. Circulation 144 (23), 1876–1890. 10.1161/CIRCULATIONAHA.120.051923 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

