The progress of molecules and strategies for the treatment of HBV infection
- PMID: 37009498
- PMCID: PMC10053227
- DOI: 10.3389/fcimb.2023.1128807
The progress of molecules and strategies for the treatment of HBV infection
Abstract
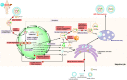
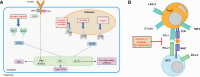
Hepatitis B virus infections have always been associated with high levels of mortality. In 2019, hepatitis B virus (HBV)-related diseases resulted in approximately 555,000 deaths globally. In view of its high lethality, the treatment of HBV infections has always presented a huge challenge. The World Health Organization (WHO) came up with ambitious targets for the elimination of hepatitis B as a major public health threat by 2030. To accomplish this goal, one of the WHO's strategies is to develop curative treatments for HBV infections. Current treatments in a clinical setting included 1 year of pegylated interferon alpha (PEG-IFNα) and long-term nucleoside analogues (NAs). Although both treatments have demonstrated outstanding antiviral effects, it has been difficult to develop a cure for HBV. The reason for this is that covalently closed circular DNA (cccDNA), integrated HBV DNA, the high viral burden, and the impaired host immune responses all hinder the development of a cure for HBV. To overcome these problems, there are clinical trials on a number of antiviral molecules being carried out, all -showing promising results so far. In this review, we summarize the functions and mechanisms of action of various synthetic molecules, natural products, traditional Chinese herbal medicines, as clustered regularly interspaced short palindromic repeats and their associated proteins (CRISPR/Cas)-based systems, zinc finger nucleases (ZFNs), and transcription activator-like effector nucleases (TALENs), all of which could destroy the stability of the HBV life cycle. In addition, we discuss the functions of immune modulators, which can enhance or activate the host immune system, as well some representative natural products with anti-HBV effects.
Keywords: HBV; HBV life cycle; inhibitors; molecules; treatment.
Copyright © 2023 Pan, Xia, He, Zeng, Shen and Huang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



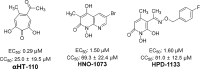

Similar articles
-
Current Status and Challenges in Anti-Hepatitis B Virus Agents Based on Inactivation/Inhibition or Elimination of Hepatitis B Virus Covalently Closed Circular DNA.Viruses. 2023 Nov 25;15(12):2315. doi: 10.3390/v15122315. Viruses. 2023. PMID: 38140556 Free PMC article. Review.
-
Orthologous CRISPR/Cas9 systems for specific and efficient degradation of covalently closed circular DNA of hepatitis B virus.Cell Mol Life Sci. 2019 May;76(9):1779-1794. doi: 10.1007/s00018-019-03021-8. Epub 2019 Jan 23. Cell Mol Life Sci. 2019. PMID: 30673820 Free PMC article.
-
Moving Fast Toward Hepatitis B Virus Elimination.Adv Exp Med Biol. 2021;1322:115-138. doi: 10.1007/978-981-16-0267-2_5. Adv Exp Med Biol. 2021. PMID: 34258739 Free PMC article.
-
Novel therapeutic approaches for hepatitis B virus covalently closed circular DNA.World J Gastroenterol. 2015 Jun 21;21(23):7084-8. doi: 10.3748/wjg.v21.i23.7084. World J Gastroenterol. 2015. PMID: 26109795 Free PMC article. Review.
-
Interferon-inducible MX2 is a host restriction factor of hepatitis B virus replication.J Hepatol. 2020 May;72(5):865-876. doi: 10.1016/j.jhep.2019.12.009. Epub 2019 Dec 18. J Hepatol. 2020. PMID: 31863794
Cited by
-
Prospects for Controlling Hepatitis B Globally.Pathogens. 2024 Mar 29;13(4):291. doi: 10.3390/pathogens13040291. Pathogens. 2024. PMID: 38668246 Free PMC article. Review.
-
Potential Benefits of In Silico Methods: A Promising Alternative in Natural Compound's Drug Discovery and Repurposing for HBV Therapy.Pharmaceuticals (Basel). 2025 Mar 16;18(3):419. doi: 10.3390/ph18030419. Pharmaceuticals (Basel). 2025. PMID: 40143195 Free PMC article. Review.
-
A scalable ultra-long-acting tenofovir phosphonate prodrug sustains HBV suppression.Sci Adv. 2025 Aug;11(31):eadw2286. doi: 10.1126/sciadv.adw2286. Epub 2025 Aug 1. Sci Adv. 2025. PMID: 40749058 Free PMC article.
-
Pathogenesis, prevention, and therapeutic advances in hepatitis B, C, and D.Virol J. 2025 Aug 11;22(1):274. doi: 10.1186/s12985-025-02907-3. Virol J. 2025. PMID: 40790753 Free PMC article. Review.
-
A Systematic Review of the Impact of Resveratrol on Viral Hepatitis and Chronic Viral Hepatitis-related Hepatocellular Carcinoma.Curr Mol Med. 2025;25(5):589-604. doi: 10.2174/0115665240284347240125072555. Curr Mol Med. 2025. PMID: 38375839
References
-
- Amin O. E., Colbeck E. J., Daffis S., Khan S., Ramakrishnan D., Pattabiraman D., et al. . (2021). Therapeutic potential of TLR8 agonist GS-9688 (Selgantolimod) in chronic hepatitis b: Remodeling of antiviral and regulatory mediators. Hepatology 74 (1), 55–71. doi: 10.1002/hep.31695 - DOI - PMC - PubMed
-
- Bazinet M., Pântea V., Placinta G., Moscalu I., Cebotarescu V., Cojuhari L., et al. . (2020). Safety and efficacy of 48 weeks REP 2139 or REP 2165, tenofovir disoproxil, and pegylated interferon Alfa-2a in patients with chronic HBV infection naïve to nucleos(t)ide therapy. Gastroenterology 158 (8), 2180–2194. doi: 10.1053/j.gastro.2020.02.058 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

