Donor-acceptor Stenhouse adduct functionalised polymer microspheres
- PMID: 37009639
- PMCID: PMC10043757
- DOI: 10.1039/d2py01591a
Donor-acceptor Stenhouse adduct functionalised polymer microspheres
Abstract
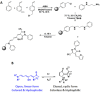
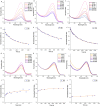
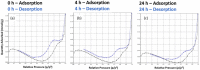
Polymers that carry donor-acceptor Stenhouse adducts (DASAs) are a very relevant class of light-responsive materials. Capable of undergoing reversible, photoinduced isomerisations under irradiation with visible light, DASAs allow for on-demand property changes to be performed in a non-invasive fashion. Applications include photothermal actuation, wavelength-selective biocatalysis, molecular capture and lithography. Typically, such functional materials incorporate DASAs either as dopants or as pendent functional groups on linear polymer chains. By contrast, the covalent incorporation of DASAs into crosslinked polymer networks is under-explored. Herein, we report DASA-functionalised crosslinked styrene-divinylbenzene-based polymer microspheres and investigate their light-induced property changes. This presents the opportunity to expand DASA-material applications into microflow assays, polymer-supported reactions and separation science. Poly(divinylbenzene-co-4-vinylbenzyl chloride-co-styrene) microspheres were prepared by precipitation polymerisation and functionalised via post-polymerisation chemical modification reactions with 3rd generation trifluoromethyl-pyrazolone DASAs to varying extents. The DASA content was verified via 19F solid-state NMR (ssNMR), and DASA switching timescales were probed by integrated sphere UV-Vis spectroscopy. Irradiation of DASA functionalised microspheres led to significant changes in their properties, notably improving their swelling in organic and aqueous environments, dispersibility in water and increasing mean particle size. This work sets the stage for future developments of light-responsive polymer supports in solid-phase extraction or phase transfer catalysis.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Gao Y. Zhang J. Liang J. Yuan D. Zhao W. Eur. Polym. J. 2022;175:111379. doi: 10.1016/j.eurpolymj.2022.111379. - DOI
-
- Fontanals N. Marcé R. M. Borrull F. Cormack P. A. G. Polym. Chem. 2015;6:7231–7244. doi: 10.1039/C5PY00771B. - DOI
-
- Saralidze K. Koole L. H. Knetsch M. L. W. Materials. 2010;3:3537–3564. doi: 10.3390/ma3063537. - DOI
-
- Kawaguchi H. Prog. Polym. Sci. 2000;25:1171–1210. doi: 10.1016/S0079-6700(00)00024-1. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
