Alirocumab and Coronary Atherosclerosis in Asymptomatic Patients with Familial Hypercholesterolemia: The ARCHITECT Study
- PMID: 37009731
- PMCID: PMC10158600
- DOI: 10.1161/CIRCULATIONAHA.122.062557
Alirocumab and Coronary Atherosclerosis in Asymptomatic Patients with Familial Hypercholesterolemia: The ARCHITECT Study
Abstract
Background: The effect of alirocumab, a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor, on coronary plaque burden in patients with familial hypercholesterolemia has not been addressed. Our aim was to assess changes in coronary plaque burden and its characteristics after treatment with alirocumab by quantification and characterization of atherosclerotic plaque throughout the coronary tree on the basis of a noninvasive analysis of coronary computed tomographic angiography in asymptomatic subjects with familial hypercholesterolemia receiving optimized and stable treatment with maximum tolerated statin dose with or without ezetimibe.
Methods: This study is a phase IV, open-label, multicenter, single-arm clinical trial to assess changes in coronary plaque burden and its characteristics after 78 weeks of treatment with alirocumab in patients with familial hypercholesterolemia without clinical atherosclerotic cardiovascular disease. Participants underwent an initial coronary computed tomographic angiography at baseline and another at 78 weeks. Every patient received 150 mg of alirocumab subcutaneiously every 14 days in addition to high-intensity statin therapy. The main outcome was the change on coronary plaque burden and its characteristics by quantification and characterization of atherosclerotic plaque throughout the coronary tree on the basis of analysis of coronary computed tomographic angiography.
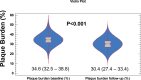
Results: The study was completed by 104 patients. The median age was 53.3 (46.2-59.4) years. Of these patients, 54 were women (51.9%). Median low-density lipoprotein cholesterol was 138.9 (117.5-175.3) mg/dL at entry and 45.0 (36.0-65.0) mg/dL at follow-up (P<0.001). Coronary plaque burden changed from 34.6% (32.5%-36.8%) at entry to 30.4% (27.4%-33.4%) at follow-up (P<0.001). A significant change in the characteristics of the coronary atherosclerosis was also found: an increase in the proportion of calcified (+0.3%; P<0.001) and mainly fibrous (+6.2%; P<0.001) plaque, accompanied by a decrease in the percentage of fibro-fatty (-3.9%; P<0.001) and necrotic plaque (-0.6%; P<0.001).
Conclusions: Treatment with alirocumab in addition to high-intensity statin therapy resulted in significant regression of coronary plaque burden and plaque stabilization on coronary computed tomographic angiography over 78 weeks in these groups of patients with familial hypercholesterolemia without clinical atherosclerotic cardiovascular disease. ARCHITECT (Effect of Alirocumab on Atherosclerotic Plaque Volume, Architecture and Composition) could link and explain ODYSSEY OUTCOMES (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) results.
Registration: URL: https://www.
Clinicaltrials: gov; Unique identifier: NCT05465278.
Keywords: alirocumab; computed tomography angiography; coronary artery disease; hypercholesterolemia; plaque, atherosclerotic.
Conflict of interest statement
Figures
Comment in
-
Letter by Long and Jiang Regarding Article, "Alirocumab and Coronary Atherosclerosis in Asymptomatic Patients With Familial Hypercholesterolemia: The ARCHITECT Study".Circulation. 2023 Sep 26;148(13):1058. doi: 10.1161/CIRCULATIONAHA.123.065391. Epub 2023 Sep 25. Circulation. 2023. PMID: 37747950 No abstract available.
-
Letter by Aldana-Bitar et al Regarding Article, "Alirocumab and Coronary Atherosclerosis in Asymptomatic Patients With Familial Hypercholesterolemia: The ARCHITECT Study".Circulation. 2023 Sep 26;148(13):1057. doi: 10.1161/CIRCULATIONAHA.123.065198. Epub 2023 Sep 25. Circulation. 2023. PMID: 37747953 No abstract available.
References
-
- Watts GF, Gidding SS, Mata P, Pang J, Sullivan DR, Yamashita S, Raal FJ, Santos RD, Ray KK. Familial hypercholesterolaemia: evolving knowledge for designing adaptive models of care. Nat Rev Cardiol. 2020;17:360–377. doi: 10.1038/s41569-019-0325-8 - PubMed
-
- Kastelein JJP, Ginsberg HN, Langslet G, Kees Hovingh G, Ceska R, Dufour R, Blom D, Civeira F, Krempf M, Lorenzato C, et al. . ODYSSEY FH i and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J. 2015;36:2996–3003. doi: 10.1093/eurheartj/ehv370 - PMC - PubMed
-
- Alonso R, Muñiz-Grijalvo O, Díaz-Díaz JL, Zambón D, de Andrés R, Arroyo-Olivares R, Fuentes-Jimenez F, Muñoz-Torrero JS, Cepeda J, Aguado R, et al. ; SAFEHEART investigators. Efficacy of PCSK9 inhibitors in the treatment of heterozygous familial hypercholesterolemia: A clinical practice experience. J Clin Lipidol. 2021;15:584–592. doi: 10.1016/j.jacl.2021.04.011 - PubMed
-
- Räber L, Ueki Y, Otsuka T, Losdat S, Häner JD, Lonborg J, Fahrni G, Iglesias JF, van Geuns R-J, Ondracek AS, et al. ; PACMAN-AMI collaborators. Effect of alirocumab added to high-intensity statin therapy on coronary atherosclerosis in patients with acute myocardial infarction. JAMA. 2022;327:1771–1781. doi: 10.1001/jama.2022.5218 - PMC - PubMed
-
- Pérez de Isla L, Alonso R, Muñiz-Grijalvo O, Díaz-Díaz JL, Zambón D, Miramontes JP, Fuentes F, Gómez de Diego JJ, González-Estrada A, Mata N, et al. . Coronary computed tomographic angiography findings and their therapeutic implications in asymptomatic patients with familial hypercholesterolemia. Lessons from the SAFEHEART study. J Clin Lipidol. 2018;12:948–957. doi: 10.1016/j.jacl.2018.04.003 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous