Evogliptin, a DPP-4 inhibitor, prevents diabetic cardiomyopathy by alleviating cardiac lipotoxicity in db/db mice
- PMID: 37009790
- PMCID: PMC10167305
- DOI: 10.1038/s12276-023-00958-6
Evogliptin, a DPP-4 inhibitor, prevents diabetic cardiomyopathy by alleviating cardiac lipotoxicity in db/db mice
Abstract
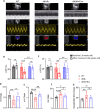
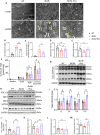
Dipeptidyl peptidase-4 (DPP-4) inhibitors are glucose-lowering drugs for type 2 diabetes mellitus (T2DM). We investigated whether evogliptin® (EVO), a DPP-4 inhibitor, could protect against diabetic cardiomyopathy (DCM) and the underlying mechanisms. Eight-week-old diabetic and obese db/db mice were administered EVO (100 mg/kg/day) daily by oral gavage for 12 weeks. db/db control mice and C57BLKS/J as wild-type (WT) mice received equal amounts of the vehicle. In addition to the hypoglycemic effect, we examined the improvement in cardiac contraction/relaxation ability, cardiac fibrosis, and myocardial hypertrophy by EVO treatment. To identify the mechanisms underlying the improvement in diabetic cardiomyopathy by EVO treatment, its effect on lipotoxicity and the mitochondrial damage caused by lipid droplet accumulation in the myocardium were analyzed. EVO lowered the blood glucose and HbA1c levels and improved insulin sensitivity but did not affect the body weight or blood lipid profile. Cardiac systolic/diastolic function, hypertrophy, and fibrosis were improved in the EVO-treated group. EVO prevented cardiac lipotoxicity by reducing the accumulation of lipid droplets in the myocardium through suppression of CD36, ACSL1, FABP3, PPARgamma, and DGAT1 and enhancement of the phosphorylation of FOXO1, indicating its inhibition. The EVO-mediated improvement in mitochondrial function and reduction in damage were achieved through activation of PGC1a/NRF1/TFAM, which activates mitochondrial biogenesis. RNA-seq results for the whole heart confirmed that EVO treatment mainly affected the differentially expressed genes (DEGs) related to lipid metabolism. Collectively, these findings demonstrate that EVO improves cardiac function by reducing lipotoxicity and mitochondrial injury and provides a potential therapeutic option for DCM.
© 2023. The Author(s).
Conflict of interest statement
This study was supported by a research fund from the Dong-A ST R&D Center (2019).
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

