A simple method to make, trap and deform a vesicle in a gel
- PMID: 37009808
- PMCID: PMC10068607
- DOI: 10.1038/s41598-023-31996-9
A simple method to make, trap and deform a vesicle in a gel
Abstract
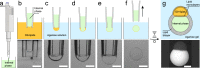
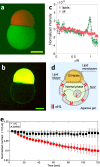
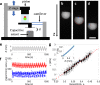
We present a simple method to produce giant lipid pseudo-vesicles (vesicles with an oily cap on the top), trapped in an agarose gel. The method can be implemented using only a regular micropipette and relies on the formation of a water/oil/water double droplet in liquid agarose. We characterize the produced vesicle with fluorescence imaging and establish the presence and integrity of the lipid bilayer by the successful insertion of [Formula: see text]-Hemolysin transmembrane proteins. Finally, we show that the vesicle can be easily mechanically deformed, non-intrusively, by indenting the surface of the gel.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Wang X, et al. Versatile phospholipid assemblies for functional synthetic cells and artificial tissues. Adv. Mater. 2021;33:1. - PubMed
-
- Jeong S, Nguyen HT, Kim CH, Ly MN, Shin K. Toward artificial cells: novel advances in energy conversion and cellular motility. Adv. Funct. Mater. 2020;30:1907182. doi: 10.1002/adfm.201907182. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

