The roles of dietary lipids and lipidomics in gut-brain axis in type 2 diabetes mellitus
- PMID: 37009872
- PMCID: PMC10068184
- DOI: 10.1186/s12967-023-04088-5
The roles of dietary lipids and lipidomics in gut-brain axis in type 2 diabetes mellitus
Abstract
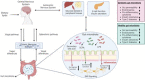
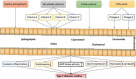
Type 2 diabetes mellitus (T2DM), one of the main types of Noncommunicable diseases (NCDs), is a systemic inflammatory disease characterized by dysfunctional pancreatic β-cells and/or peripheral insulin resistance, resulting in impaired glucose and lipid metabolism. Genetic, metabolic, multiple lifestyle, and sociodemographic factors are known as related to high T2DM risk. Dietary lipids and lipid metabolism are significant metabolic modulators in T2DM and T2DM-related complications. Besides, accumulated evidence suggests that altered gut microbiota which plays an important role in the metabolic health of the host contributes significantly to T2DM involving impaired or improved glucose and lipid metabolism. At this point, dietary lipids may affect host physiology and health via interaction with the gut microbiota. Besides, increasing evidence in the literature suggests that lipidomics as novel parameters detected with holistic analytical techniques have important roles in the pathogenesis and progression of T2DM, through various mechanisms of action including gut-brain axis modulation. A better understanding of the roles of some nutrients and lipidomics in T2DM through gut microbiota interactions will help develop new strategies for the prevention and treatment of T2DM. However, this issue has not yet been entirely discussed in the literature. The present review provides up-to-date knowledge on the roles of dietary lipids and lipidomics in gut-brain axis in T2DM and some nutritional strategies in T2DM considering lipids- lipidomics and gut microbiota interactions are given.
Keywords: Diet; Gut microbiota; Lipidomics; Lipids; Type 2 diabetes mellitus.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Paulson KR, Kamath AM, Alam T, Bienhoff K, Abady GG, Abbas J, et al. Global, regional, and national progress towards Sustainable Development Goal 3.2 for neonatal and child health: all-cause and cause-specific mortality findings from the Global Burden of Disease Study 2019. Lancet. 2021;398(10303):870–905. doi: 10.1016/S0140-6736(21)01207-1. - DOI - PMC - PubMed
-
- American Diabetes Association Professional Practice Committee 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes—2022. Diabetes Care. 2022;45:17–38. doi: 10.2337/dc22-S002. - DOI
-
- International Diabetes Federation. IDF Diabetes Atlas 2021. https://diabetesatlas.org/atlas/tenth-edition/ Accessed 11 November 2022
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

