Cancer-associated fibroblast-secreted glucosamine alters the androgen biosynthesis program in prostate cancer via HSD3B1 upregulation
- PMID: 37009898
- PMCID: PMC10065083
- DOI: 10.1172/JCI161913
Cancer-associated fibroblast-secreted glucosamine alters the androgen biosynthesis program in prostate cancer via HSD3B1 upregulation
Abstract
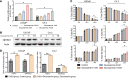
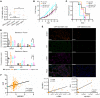
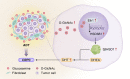
After androgen deprivation, prostate cancer frequently becomes castration resistant (CRPC), with intratumoral androgen production from extragonadal precursors that activate the androgen receptor pathway. 3β-Hydroxysteroid dehydrogenase-1 (3βHSD1) is the rate-limiting enzyme for extragonadal androgen synthesis, which together lead to CRPC. Here, we show that cancer-associated fibroblasts (CAFs) increased epithelial 3βHSD1 expression, induced androgen synthesis, activated the androgen receptor, and induced CRPC. Unbiased metabolomics revealed that CAF-secreted glucosamine specifically induced 3βHSD1. CAFs induced higher GlcNAcylation in cancer cells and elevated expression of the transcription factor Elk1, which induced higher 3βHSD1 expression and activity. Elk1 genetic ablation in cancer epithelial cells suppressed CAF-induced androgen biosynthesis in vivo. In patient samples, multiplex fluorescent imaging showed that tumor cells expressed more 3βHSD1 and Elk1 in CAF-enriched areas compared with CAF-deficient areas. Our findings suggest that CAF-secreted glucosamine increases GlcNAcylation in prostate cancer cells, promoting Elk1-induced HSD3B1 transcription, which upregulates de novo intratumoral androgen synthesis to overcome castration.
Keywords: Oncology; Prostate cancer; Sex hormones.
Conflict of interest statement
Figures



Comment in
-
CAFs promote CRPC.Nat Rev Cancer. 2023 Jun;23(6):349. doi: 10.1038/s41568-023-00587-1. Nat Rev Cancer. 2023. PMID: 37173415 No abstract available.
-
Uro-Science.J Urol. 2023 Dec;210(6):922-924. doi: 10.1097/JU.0000000000003662. Epub 2023 Sep 29. J Urol. 2023. PMID: 37774378 No abstract available.
Similar articles
-
BMX controls 3βHSD1 and sex steroid biosynthesis in cancer.J Clin Invest. 2023 Jan 17;133(2):e163498. doi: 10.1172/JCI163498. J Clin Invest. 2023. PMID: 36647826 Free PMC article.
-
AR Signaling in Prostate Cancer Regulates a Feed-Forward Mechanism of Androgen Synthesis by Way of HSD3B1 Upregulation.Endocrinology. 2018 Aug 1;159(8):2884-2890. doi: 10.1210/en.2018-00283. Endocrinology. 2018. PMID: 29850791 Free PMC article.
-
Castration-resistant prostate cancer cells are dependent on the high activity of CDK7.J Cancer Res Clin Oncol. 2023 Jul;149(8):5255-5263. doi: 10.1007/s00432-022-04475-3. Epub 2022 Nov 18. J Cancer Res Clin Oncol. 2023. PMID: 36401094 Free PMC article.
-
EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer.Eur Urol. 2014 Feb;65(2):467-79. doi: 10.1016/j.eururo.2013.11.002. Epub 2013 Nov 12. Eur Urol. 2014. PMID: 24321502
-
Efficacy of Systemic Treatment in Prostate Cancer Patients With Visceral Metastasis: A Systematic Review, Meta-analysis, and Network Meta-analysis.J Urol. 2023 Sep;210(3):416-429. doi: 10.1097/JU.0000000000003594. Epub 2023 Jun 20. J Urol. 2023. PMID: 37339479
Cited by
-
The Mechanism by Which Hedgehog Interacting Protein (HHIP) in Cancer-Associated Fibroblasts Regulate the Secretion of Inflammatory Factors Through the JAK1/STAT3 Pathway Affecting Prostate Cancer Stemness.J Inflamm Res. 2024 Nov 11;17:8659-8680. doi: 10.2147/JIR.S472124. eCollection 2024. J Inflamm Res. 2024. PMID: 39553307 Free PMC article.
-
The multifaceted role of the stroma in the healthy prostate and prostate cancer.J Transl Med. 2024 Sep 5;22(1):825. doi: 10.1186/s12967-024-05564-2. J Transl Med. 2024. PMID: 39238004 Free PMC article. Review.
-
Survival of men with metastatic hormone-sensitive prostate cancer and adrenal-permissive HSD3B1 inheritance.J Clin Invest. 2024 Sep 17;134(18):e183583. doi: 10.1172/JCI183583. J Clin Invest. 2024. PMID: 39286977 Free PMC article.
-
Treatment-induced stemness and lineage plasticity in driving prostate cancer therapy resistance.Cancer Heterog Plast. 2024;1(1):0005. doi: 10.47248/chp2401010005. Epub 2024 Aug 25. Cancer Heterog Plast. 2024. PMID: 39363904 Free PMC article.
-
Role of exosomes in castration-resistant prostate cancer.Front Oncol. 2025 May 14;15:1498733. doi: 10.3389/fonc.2025.1498733. eCollection 2025. Front Oncol. 2025. PMID: 40438694 Free PMC article. Review.

