Characterisation of infection-induced SARS-CoV-2 seroprevalence amongst children and adolescents in North Carolina
- PMID: 37009915
- PMCID: PMC10154644
- DOI: 10.1017/S0950268823000481
Characterisation of infection-induced SARS-CoV-2 seroprevalence amongst children and adolescents in North Carolina
Abstract
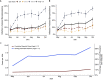
Few prospective studies have documented the seropositivity among those children infected with severe acute respiratory syndrome coronavirus 2. From 2 April 2021 to 24 June 2021, we prospectively enrolled children between the ages of 2 and 17 years at three North Carolina healthcare systems. Participants received at least four at-home serological tests detecting the presence of antibodies against, but not differentiating between, the nucleocapsid or spike antigen. A total of 1,058 participants were enrolled in the study, completing 2,709 tests between 1 May 2021 and 31 October 2021. Using multilevel regression with poststratification techniques and considering our assay sensitivity and sensitivity, we estimated that the seroprevalence of infection-induced antibodies among unvaccinated children and adolescents aged 2-17 years in North Carolina increased from 15.2% (95% credible interval, CrI 9.0-22.0) in May 2021 to 54.1% (95% CrI 46.7-61.1) by October 2021, indicating an average infection-to-reported-case ratio of 5. A rapid rise in seropositivity was most pronounced in those unvaccinated children aged 12-17 years, based on our estimates. This study underlines the utility of serial, serological testing to inform a broader understanding of the regional immune landscape and spread of infection.
Trial registration: ClinicalTrials.gov NCT04342884.
Keywords: Adolescent; COVID-19; SARS-CoV-2; child; paediatrics; seroepidemiologic studies; serology.
Conflict of interest statement
The authors have no relevant conflicts of interest to disclose.
Figures


References
-
- Spychalski P, Błażyńska-Spychalska A, Kobiela J (2020). Estimating case fatality rates of COVID-19. Lancet Infectious Diseases 20(7), 774–775 [cited 2020 December 31]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7270730/. - PMC - PubMed
-
- Meyerowitz-Katz G, Merone L (2020). A systematic review and meta-analysis of published research data on COVID-19 infection fatality rates. International Journal of Infectious Diseases 101, 138–148 [cited 2020 December 31]. Available from: http://www.sciencedirect.com/science/article/pii/S1201971220321809. - PMC - PubMed
-
- Viner RM et al. (2020). Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: a systematic review and meta-analysis. JAMA Pediatrics 175, 143–156 [cited 2021 January 6]. Available from: https://jamanetwork.com/journals/jamapediatrics/fullarticle/2771181. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

