Single-cell RNA-seq of heart reveals intercellular communication drivers of myocardial fibrosis in diabetic cardiomyopathy
- PMID: 37010266
- PMCID: PMC10110238
- DOI: 10.7554/eLife.80479
Single-cell RNA-seq of heart reveals intercellular communication drivers of myocardial fibrosis in diabetic cardiomyopathy
Abstract
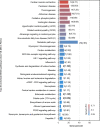
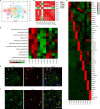
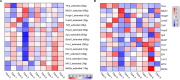
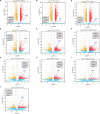
Myocardial fibrosis is the characteristic pathology of diabetes-induced cardiomyopathy. Therefore, an in-depth study of cardiac heterogeneity and cell-to-cell interactions can help elucidate the pathogenesis of diabetic myocardial fibrosis and identify treatment targets for the treatment of this disease. In this study, we investigated intercellular communication drivers of myocardial fibrosis in mouse heart with high-fat-diet/streptozotocin-induced diabetes at single-cell resolution. Intercellular and protein-protein interaction networks of fibroblasts and macrophages, endothelial cells, as well as fibroblasts and epicardial cells revealed critical changes in ligand-receptor interactions such as Pdgf(s)-Pdgfra and Efemp1-Egfr, which promote the development of a profibrotic microenvironment during the progression of and confirmed that the specific inhibition of the Pdgfra axis could significantly improve diabetic myocardial fibrosis. We also identified phenotypically distinct Hrchi and Postnhi fibroblast subpopulations associated with pathological extracellular matrix remodeling, of which the Hrchi fibroblasts were found to be the most profibrogenic under diabetic conditions. Finally, we validated the role of the Itgb1 hub gene-mediated intercellular communication drivers of diabetic myocardial fibrosis in Hrchi fibroblasts, and confirmed the results through AAV9-mediated Itgb1 knockdown in the heart of diabetic mice. In summary, cardiac cell mapping provides novel insights into intercellular communication drivers involved in pathological extracellular matrix remodeling during diabetic myocardial fibrosis.
Keywords: cell biology; diabetic cardiomyopathy; liver fibrosis; mouse; rabbit; rat; retinal regeneration.
© 2023, Li et al.
Conflict of interest statement
WL, XL, YZ, YQ, JZ, LH, ZX, SY, CW, JA, ZZ, SQ No competing interests declared
Figures

















Update of
- doi: 10.1101/2022.06.29.498129
References
-
- Aibar S, González-Blas CB, Moerman T, Huynh-Thu VA, Imrichova H, Hulselmans G, Rambow F, Marine J-C, Geurts P, Aerts J, van den Oord J, Atak ZK, Wouters J, Aerts S. SCENIC: single-cell regulatory network inference and clustering. Nature Methods. 2017;14:1083–1086. doi: 10.1038/nmeth.4463. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

